Health
Research into AIDS cure advancing but remains in ‘very early days’
HIV treatment and prevention getting ‘better and better’

Editor’s note: This is part two of our interview with Carl Dieffenbach, director of the Division of AIDS at the National Institute of Allergies and Infectious Diseases. Click here to read part one.
Unlike the coronavirus, the AIDS virus’s ability to permanently infect the human body has made it more difficult to develop an AIDS vaccine, and research into a cure for HIV/AIDS is continuing to advance but remains in its “very early days,” according to Carl W. Dieffenbach, who has served for the past 25 years as director of the National Institutes of Health’s Division of AIDS.
But in an interview with the Washington Blade, Dieffenbach, who holds a doctorate degree in biophysics, said the already highly effective antiretroviral drug treatment for HIV is continuing to advance to a point where the current one pill per day regimen may soon be replaced by a single injection that will make HIV undetectable in the body and untransmitable for six months and possibly a full year.
He said the single injection advance would be applicable for both people who are HIV positive as well as for those who are HIV negative and are taking the current one pill per day prevention medication known as PrEP.
“One of the things I am most happy with is the whole U equals U movement – that undetectable equals untransmitable,” Dieffenbach said in referring to the current antiviral medication that makes HIV undetectable in the human body and prevents the virus from being transmitted to another person through sexual relations.
“That really is a rallying cry for people living with HIV that you can become fully suppressed and live knowing that there is no virus in your body as long as you take your pill, and you are free to love,” he told the Blade. “And that’s a wonderful thing.”
Although he didn’t say so directly, Dieffenbach made it clear that he and other government and private industry researchers working on an AIDS vaccine and an HIV/AIDS cure know that people with HIV can live a full and productive life as the push for a vaccine and cure continues.
Dieffenbach said a dramatic difference in the genetic makeup between the coronavirus and the AIDS virus is the reason why an AIDS vaccine has yet to be developed after more than 20 years of vaccine research while a COVID-19 vaccine was developed in a little more than a year.
“Once a person becomes HIV positive, that individual is HIV positive for life,” he said. “There is no going back. There is no spontaneous cure.” By contrast, Dieffenbach points out that with coronavirus, just five percent of those who become infected become seriously ill and are at risk of dying. He said between 35 percent and 40 percent of those infected with coronavirus are asymptomatic and often are unaware that they were infected.
“So, the human immune system by and large does a pretty good job of fighting off the coronavirus,” he said. That, among other factors, has made it possible to develop an effective COVID vaccine sooner than an AIDS vaccine, according to Dieffenbach.
Washington Blade: Where do things stand now in the progress of developing a cure for HIV and AIDS?
Carl Dieffenbach: So, let’s talk a moment about what we are doing in the space of trying to achieve a cure for HIV. Clearly, this is one of the two major research programs or research goals remaining in HIV – an effective and durable vaccine and then a cure that allows people to not take an antiretroviral [drug] and still live the ‘U’ equals ‘U’ [undetectable equals untransmitable] life.
What we want is a cure that really allows people to be free of HIV. And that can be achieved in two ways. You could see the HIV be eliminated or eradicated from the body. You would call that a sterilizing cure. And the other would be more of an immunological or other means of control that would suppress the virus similar to the way the antiretrovirals do, but it’s using the natural immunity, the induced immunity that the human body is capable of generating.
Up until recently there hadn’t been examples of an individual that had achieved that kind of cure. Just recently there was one reported. The big program we have in cure research is called the Martin Delany Collaboratories for Cure Research. And Marty was one of the lead activists in the very early days of HIV through the ‘90s. And he really pushed NIH very, very hard to not forget about a cure and to really focus on the best possible anti-virals.
He was just a strong leader and a really wonderful person who just pushed constantly the way you would hope the activist community would continue to try to drive improvements, even when things were going well. So, we felt it was a great way to honor Marty to name the program after him. This program has been around for a little over a decade and it gets more sophisticated and better every cycle.
And the two methods I mentioned – the ability to eliminate the virus completely and establish an immunologic or some other means of control – are major themes of these programs. It’s still in the very early days. There are limited clinical trials ongoing, but they’re very exploratory. There are maybe hints of things coming in the next couple of years. But it remains in the very early days. In some ways it’s similar to where we are with vaccines where we’ve had a little bit of success but nothing really that we then can say this is the vaccine for the future.
So, these two types of research – a vaccine and cure – remain our top research priorities. And we will continue at this until we have HIV vaccines and the abilities to cure, because we cannot really control and eliminate the epidemic without either of those two strategies.
Blade: Can you talk a little about the human trials that are going on now for a possible HIV cure being conducted by the Rockville-based company American Gene Technologies?
Dieffenbach: That’s right. One approach for achieving a cure are these gene-based strategies. There is a company that has a strategy for a gene-based treatment that they have been working on for a number of years. And that has been moving forward. And the proof will be in the pudding when we have a sufficient number of people in a way that are truly evaluated.
There are also strategies that look at ways of using what amounts to scissors, molecular scissors that can go in and chop out the virus. So, there are a number of strategies that people are using or considering for this idea of elimination of the reservoir, including the gene therapy method that we were just discussing.
Blade: The company conducting the gene therapy trials has said the treatment they hope will lead to a cure requires taking blood from someone, altering the genetic makeup of certain cells, and re-infusing the blood back into their body. Is that something that would be practical for treating a large number of people?
Dieffenbach: So, all of these gene therapy strategies are in the very experimental stage. They have to do something called ex-vivo transduction. That’s fancy words for saying what you just said. You take cells out of the human body, alter them by adding the new therapeutic and incorporate it into the cell, and re-infuse those cells back into the human body. So, first you start with one cell type like fully differentiated lymphocytes and then you move on.
The ultimate goal will be to get it so you can take a shot, where the shot would go in with the gene therapy and basically go into cells and immunize the cells in such a way that they provide protection from HIV infection as well as elimination of existing copies of HIV. So, we’re many steps away from that.
Blade: Some people may be asking why a COVID vaccine has been developed in just over a year since the worldwide COVID outbreak, but an HIV vaccine has not yet been developed after 20 or more years of research. Is there something different with the coronavirus as opposed to the HIV virus that might explain why we haven’t had an HIV vaccine at this time?
Dieffenbach: I think this is a really important point. And I want to talk about two different activities. One is the differences between the viruses themselves. With coronavirus, five percent of people who become infected with coronavirus actually get sick and get into a hospital and have near death experiences. Thirty-five to 40 percent of people who get infected with coronavirus are actually never aware that they were infected.
So, the human immune system by and large does a pretty good job of fighting off the coronavirus. But it is incredibly infectious. It is spread by aerosol. With HIV, it is transmitted sexually. It’s transmitted through blood and other bodily fluids. Once a person becomes HIV positive, that individual is HIV positive for life. There is no going back. There’s no spontaneous cure. We’ve had 70 million people around the world acquire HIV. By last count, there may be one person in all the years that may have spontaneously cleared their HIV infection. That took 12 years of that person’s life.
It is a rarity. So, from that perspective the type of immunity that you need to induce by a vaccine is so fundamentally different for coronavirus and for HIV. So, that’s the first step.
The second thing is why were we so successful with the coronavirus vaccine? It wasn’t dumb luck. Going back to the earliest SARS outbreak and through MERS and through other respiratory viruses the research team here at NIH has been looking at ways of building the better mouse trap, building a better immunogen. Take a part of the virus and make it the best it could be in terms of presenting or showing itself to the human immune system so that you get an incredibly robust quality response. And that was the work that was done at the VRC, the [NIH] Vaccine Research Center.
So, when that group first published their work on what we call this stabilized spike we offered that technology to all the vaccine manufacturers. And Moderna, Pfizer, and J&J all chose to use this modified version. AstraZeneca and Oxford chose different paths. The Chinese and the Russians chose a different path. And I think the quality of the vaccine and the effectiveness of the vaccine shows in part because of the genetic engineering that we have done to make it the best immunogenetic it can be.
So, it was a two-fold thing. We built a better vaccine to tackle a disease that really natural immunity can work well on. That’s one of the reasons why our vaccines – the Moderna, the Pfizer, and the J&J are still quite active against all these variants. It’s because their immune response was so robust. So, it was probably six to ten years of work that led us to that exact moment when SARS-CV2 came along that we know what to do with this. We were able to design a vaccine based on all that previous work within a very short period of time and start clinical trials within 60 days of identifying the coronavirus sequence. It wasn’t magic. It was hard work.
That’s a great story. There are so many unsung heroes in this. And it’s a great thing to be part of that we – NIH – could make it so it wasn’t just a proprietary thing for us. But we were able to give the world a way of making the best vaccine possible and to allow the companies to pick it up and run with it. So, again, at the end of the day the vaccines that I think we’ll come back to rely upon were made with this construct that was developed here through years of research.
Blade: Is there anything I did not ask you that is relevant to the HIV research?
Dieffenbach: Well, just to close the loop, so now that we learned all those lessons from the coronavirus vaccine, we’re going back to HIV vaccines and applying some of the rules and technologies and things that we’ve learned. Now we’re going back and looking at that more carefully and trying different things. And thinking about how we can build a better HIV vaccine based on what we know for a coronavirus vaccine.
So, we’re trying to complete the cycle. We started with HIV. We developed the platforms, applied it to coronavirus. And now we’re trying to close the loop.
Blade: You’ve been saying that these clinical trials for an AIDS vaccine have been going on for a while. Do you recall when the first AIDS vaccine trial started?
Dieffenbach: The very first trial for an AIDS vaccine was done in the ‘90s. And it didn’t work. It was a single protein. It induced antibodies. But the antibody did not react with the intact viruses. So, it failed. And that was the AIDS vax experience.
Blade: Do you remember when in the ‘90s that was?
Dieffenbach: The papers were finally published in 2003. So, the studies started in the late 90s and were completed in the early 2000s.
Blade: So, it appears that happened around the time the effective anti-retroviral drugs became available?
Dieffenbach: The highly active anti-retroviral therapy first made its debut in 1995. And that was a combination of AZT, 3TC, and either Crixivan, the protease inhibitor, or a different protease inhibitor from either La Roche or Abbott. And those drugs were quite effective in preventing the virus and helping people. But they all had tremendous side-effects as you will remember. And we then got better and better and better therapies where we are now at one pill once a day.
That is my background in this. I came from the drug side working with the companies back in the early ‘90s to bring those along. And I grew up in this field and then graduated to director of AIDS and then continued on to therapy and cure and vaccines ever since. I’ve been director since 2007.
Monkeypox
US contributes more than $90 million to fight mpox outbreak in Africa
WHO and Africa CDC has declared a public health emergency
The U.S. has contributed more than $90 million to the fight against the mpox outbreak in Africa.
The U.S. Agency for International Development on Tuesday in a press release announced “up to an additional” $35 million “in emergency health assistance to bolster response efforts for the clade I mpox outbreak in Central and Eastern Africa, pending congressional notification.” The press release notes the Biden-Harris administration previously pledged more than $55 million to fight the outbreak in Congo and other African countries.
“The additional assistance announced today will enable USAID to continue working closely with affected countries, as well as regional and global health partners, to expand support and reduce the impact of this outbreak as it continues to evolve,” it reads. “USAID support includes assistance with surveillance, diagnostics, risk communication and community engagement, infection prevention and control, case management, and vaccination planning and coordination.”
The World Health Organization and the Africa Centers for Disease Control and Prevention last week declared the outbreak a public health emergency.
The Washington Blade last week reported there are more than 17,000 suspected mpox cases across in Congo, Uganda, Kenya, Rwanda, and other African countries. The outbreak has claimed more than 500 lives, mostly in Congo.
Health
Mpox outbreak in Africa declared global health emergency
ONE: 10 million vaccine doses needed on the continent

Medical facilities that provide treatment to gay and bisexual men in some East African countries are already collaborating with them to prevent the spread of a new wave of mpox cases after the World Health Organization on Wednesday declared a global health emergency.
The collaboration, both in Uganda and Kenya, comes amid WHO’s latest report released on Aug. 12, which reveals that nine out of every 10 reported mpox cases are men with sex as the most common cause of infection.
The global mpox outbreak report — based on data that national authorities collected between January 2022 and June of this year — notes 87,189 of the 90,410 reported cases were men. Ninety-six percent of whom were infected through sex.
Sexual contact as the leading mode of transmission accounted for 19,102 of 22,802 cases, followed by non-sexual person-to-person contact. Genital rash was the most common symptom, followed by fever and systemic rash.
The WHO report states the pattern of mpox virus transmission has persisted over the last six months, with 97 percent of new cases reporting sexual contact through oral, vaginal, or anal sex with infected people.
“Sexual transmission has been recorded in the Democratic Republic of Congo among sex workers and men who have sex with men,” the report reads. “Among cases exposed through sexual contact in the Democratic Republic of the Congo, some individuals present only with genital lesions, rather than the more typical extensive rash associated with the virus.”
The growing mpox cases, which are now more than 2,800 reported cases in at least 13 African countries that include Kenya, Uganda, Rwanda, and prompted the Africa Centers for Disease Control and Prevention this week to declare the disease a public health emergency for resource mobilization on the continent to tackle it.
“Africa has long been on the frontlines in the fight against infectious diseases, often with limited resources,” said Africa CDC Director General Jean Kaseya. “The battle against Mpox demands a global response. We need your support, expertise, and solidarity. The world cannot afford to turn a blind eye to this crisis.”
The disease has so far claimed more than 500 lives, mostly in Congo, even as the Africa CDC notes suspected mpox cases across the continent have surged past 17,000, compared to 7,146 cases in 2022 and 14,957 cases last year.
“This is just the tip of the iceberg when we consider the many weaknesses in surveillance, laboratory testing, and contact tracing,” Kaseya said.
WHO, led by Director General Tedros Adhanom Ghebreyesus, also followed the Africa CDC’s move by declaring the mpox outbreak a public health emergency of international concern.
The latest WHO report reveals that men, including those who identify as gay and bisexual, constitute most mpox cases in Kenya and Uganda. The two countries have recorded their first cases, and has put queer rights organizations and health care centers that treat the LGBTQ community on high alert.
The Uganda Minority Shelters Consortium, for example, confirmed to the Washington Blade that the collaboration with health service providers to prevent the spread of mpox among gay and bisexual men is “nascent and uneven.”
“While some community-led health service providers such as Ark Wellness Clinic, Children of the Sun Clinic, Ice Breakers Uganda Clinic, and Happy Family Youth Clinic, have demonstrated commendable efforts, widespread collaboration on mpox prevention remains a significant gap,” UMSC Coordinator John Grace stated. “This is particularly evident when compared to the response to the previous Red Eyes outbreak within the LGBT community.”
Grace noted that as of Wednesday, there were no known queer-friendly health service providers to offer mpox vaccinations to men who have sex with men. He called for health care centers to provide inclusive services and a more coordinated approach.
Although Grace pointed out the fear of discrimination — and particularly Uganda’s Anti-Homosexuality Act — remains a big barrier to mpox prevention through testing, vaccination, and treatment among queer people, he confirmed no mpox cases have been reported among the LGBTQ community.
Uganda so far has reported two mpox cases — refugees who had travelled from Congo.
“We are for the most part encouraging safer sex practices even after potential future vaccinations are conducted as it can also be spread through bodily fluids like saliva and sweat,” Grace said.
Grace also noted that raising awareness about mpox among the queer community and seeking treatment when infected remains a challenge due to the historical and ongoing homophobic stigma and that more comprehensive and reliable advocacy is needed. He said Grindr and other digital platforms have been crucial in raising awareness.
The declarations of mpox as a global health emergency have already attracted demand for global leaders to support African countries to swiftly obtain the necessary vaccines and diagnostics.
“History shows we must act quickly and decisively when a public health emergency strikes. The current Mpox outbreak in Africa is one such emergency,” said ONE Global Health Senior Policy Director Jenny Ottenhoff.
ONE is a global, nonpartisan organization that advocates for the investments needed to create economic opportunities and healthier lives in Africa.
Ottenhoff warned failure to support the African countries with medical supplies needed to tackle mpox would leave the continent defenseless against the virus.
To ensure that African countries are adequately supported, ONE wants governments and pharmaceutical companies to urgently increase the provision of mpox vaccines so that the most affected African countries have affordable access to them. It also notes 10 million vaccine doses are currently needed to control the mpox outbreak in Africa, yet the continent has only 200,000 doses.
The Blade has reached out to Ishtar MSM, a community-based healthcare center in Nairobi, Kenya, that offers to service to gay and bisexual men, about their response to the mpox outbreak.
Health
White House urged to expand PrEP coverage for injectable form
HIV/AIDS service organizations made call on Wednesday
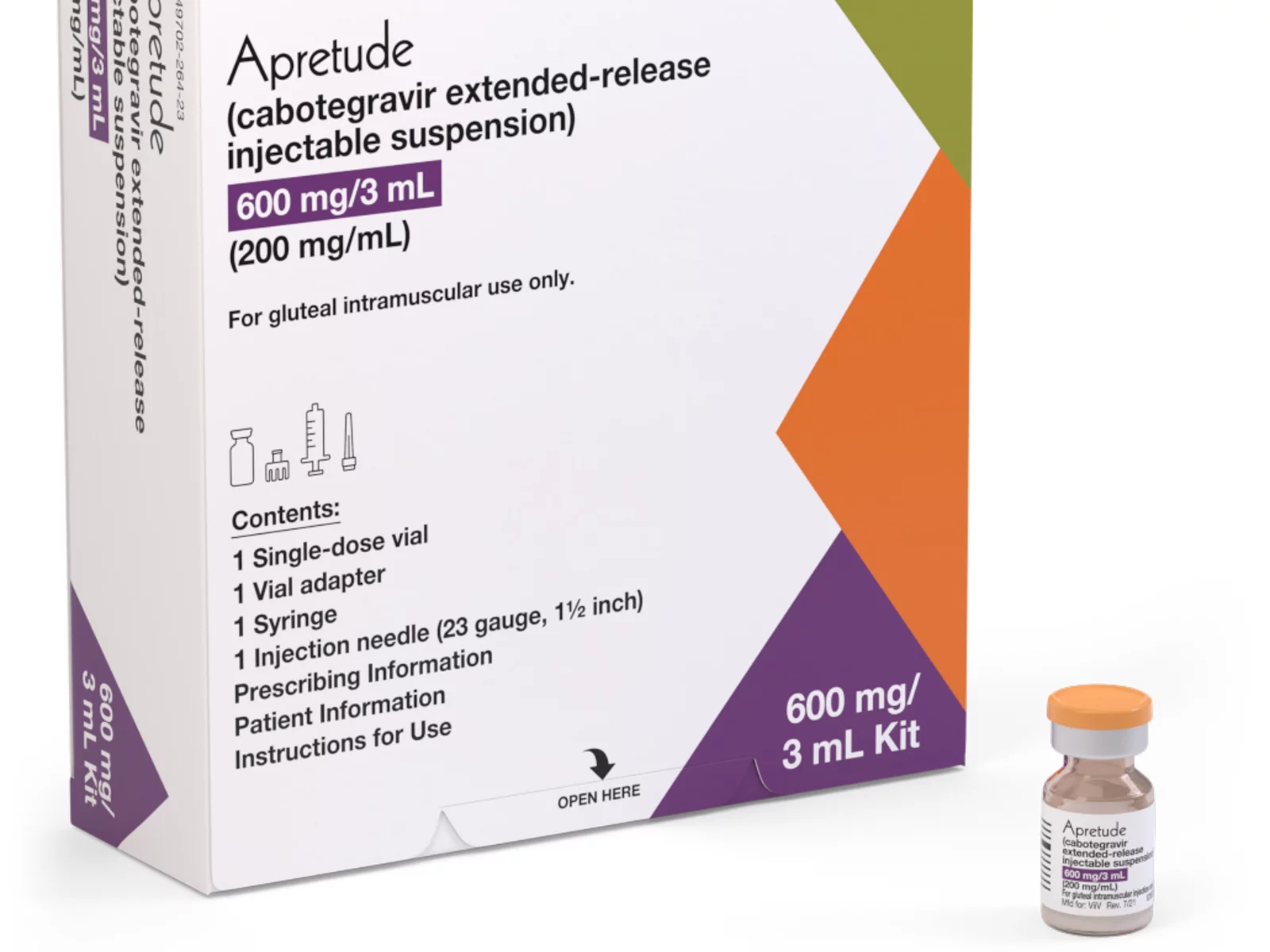
A coalition of 63 organizations dedicated to ending HIV called on the Biden-Harris administration on Wednesday to require insurers to cover long-acting pre-exposure prophylaxis (PrEP) without cost-sharing.
In a letter to Chiquita Brooks-LaSure, administrator of the Centers for Medicare and Medicaid Services, the groups emphasized the need for broad and equitable access to PrEP free of insurance barriers.
Long-acting PrEP is an injectable form of PrEP that’s effective over a long period of time. The FDA approved Apretude (cabotegravir extended-release injectable suspension) as the first and only long-acting injectable PrEP in late 2021. It’s intended for adults and adolescents weighing at least 77 lbs. who are at risk for HIV through sex.
The U.S. Preventive Services Task Force updated its recommendation for PrEP on Aug. 22, 2023, to include new medications such as the first long-acting PrEP drug. The coalition wants CMS to issue guidance requiring insurers to cover all forms of PrEP, including current and future FDA-approved drugs.
“Long-acting PrEP can be the answer to low PrEP uptake, particularly in communities not using PrEP today,” said Carl Schmid, executive director of the HIV+Hepatitis Policy Institute. “The Biden administration has an opportunity to ensure that people with private insurance can access PrEP now and into the future, free of any cost-sharing, with properly worded guidance to insurers.”
Currently, only 36 percent of those who could benefit from PrEP are using it. Significant disparities exist among racial and ethnic groups. Black people constitute 39 percent of new HIV diagnoses but only 14 percent of PrEP users, while Latinos represent 31 percent of new diagnoses but only 18 percent of PrEP users. In contrast, white people represent 24 percent of HIV diagnoses but 64 percent of PrEP users.
The groups also want CMS to prohibit insurers from employing prior authorization for PrEP, citing it as a significant barrier to access. Several states, including New York and California, already prohibit prior authorization for PrEP.
Modeling conducted for HIV+Hep, based on clinical trials of a once every 2-month injection, suggests that 87 percent more HIV cases would be averted compared to daily oral PrEP, with $4.25 billion in averted healthcare costs over 10 years.
Despite guidance issued to insurers in July 2021, PrEP users continue to report being charged cost-sharing for both the drug and ancillary services. A recent review of claims data found that 36 percent of PrEP users were charged for their drugs, and even 31 percent of those using generic PrEP faced cost-sharing.
The coalition’s letter follows a more detailed communication sent by HIV+Hepatitis Policy Institute to the Biden administration on July 2.
Signatories to the community letter include Advocates for Youth, AIDS United, Equality California, Fenway Health, Human Rights Campaign, and the National Coalition of STD Directors, among others.




















