National
HIV research sped development of COVID vaccine
Top NIH official says success in coronavirus will boost AIDS work

Since 1996, Carl W. Dieffenbach, who holds a Ph.D. in biophysics from John Hopkins University, has served as director of the Division of AIDS at the National Institute of Allergies and Infectious Diseases, which is an arm of the U.S. National Institutes of Health or NIH.
In a June 10 interview with the Washington Blade, Dieffenbach gave an update on the extensive, ongoing research into the development of an HIV/AIDS vaccine that he has helped to coordinate for many years, including current human trials for a prospective AIDS vaccine taking place in the U.S., South America, and Africa.
One thing he feels passionate about is a development not widely reported in the media reports about the successful development of the COVID-19 vaccine. According to Dieffenbach, the extensive research into an AIDS vaccine in recent and past years, while not yet successful in yielding an effective AIDS vaccine, helped lay the groundwork for the rapid development of the different versions of a COVID vaccine.
“Because my division runs the largest clinical trials program in the word, we jumped in with both feet to help with coronavirus disease for both vaccines and drugs and things like that,” he said. “And the platforms that were used – the way they are making the coronavirus vaccines – the RNA vaccines with Moderna – were first piloted by NIH and Moderna to try to make an HIV vaccine,” Dieffenbach says.
“So, in many ways, the work for the past 25 years that we’ve done in HIV vaccines sped the development of coronavirus vaccines,” he told the Blade. “And now it’s time to take what we’ve learned from coronavirus and take it back to HIV and start afresh or continue with what we have and build upon from what we have learned.”
Dieffenbach says one reason the development of a COVID vaccine came about before an AIDS vaccine, despite more than 20 years of AIDS vaccine research, is that the HIV virus is far more complex than the coronavirus, especially its ability to infect and remain embedded in the infected person for life.
“Back in 2007 we had the first hint that an AIDS vaccine might be possible with a study called RV144,” Dieffenbach says. “We spent 10 years trying to replicate that, and we just completed that study – a study called HVTN702. And it showed no efficacy,” he said, meaning it did not work.
“So that was a big disappointment to us,” he says “But in the meantime, we had pushed forward with the J&J [Johnson and Johnson pharmaceutical company] vaccine and are pretty far along. We’ll see what happens. We should know in the next several months whether the N26 version of an AIDS vaccine, and HIV vaccine works or not,” he says. “We’re very close to an answer.”
Washington Blade: Where do things stand in the development of an HIV/AIDS vaccine in light of Dr. Fauci’s statement a few weeks ago that the development of a COVID-19 vaccine could provide a boost to developing an AIDS vaccine?
Carl Dieffenbach: Sure. So, maybe I can start by introducing myself to you as a way of putting this into a context.
So, I’m the director of the Division of AIDS, which is the largest funder of HIV research in the world. And I report directly to Dr. Fauci. So, I’m responsible for all AIDS, all the time. And that is my passion and purpose in life. Part of that is working toward a safe, effective, and durable HIV vaccine, which has been one of the two most challenging questions left in science today. The other is a cure. They are connected in some ways.
So, with that as background, when coronavirus disease came along – because my division runs the largest clinical trials program in the world – we jumped in with both feet to help with coronavirus disease for both vaccines and drugs and things like that. And the platforms that were used – the way they are making the coronavirus vaccines – the RNA vaccines with Moderna were first piloted by NIH and Moderna to try to make an HIV vaccine. So, we’ve being working on that platform with Moderna for several years.
The leadership at Pfizer used to be part of a group at Penn, where we were also working with them. The J&J vaccine – we currently have in two Phase III clinical trials for HIV, one in sub-Saharan Africa, specifically in young women and the other one in the Americas in men who have sex with men and transgender individuals. Both of those Phase IIIs are moving along. The women’s study is fully enrolled. The men’s study was hit hard by COVID, but we worked through and will be fully enrolled by September.
One other vaccine just to talk about is the Oxford vaccine, the AstraZeneca vaccine. That is also using a platform at Oxford University, which has been used for HIV. So, in many ways, the work for the past 25 years that we’ve done in HIV vaccines sped the development of coronavirus vaccines. And now it’s time to take what we’ve learned from coronavirus and take it back to HIV and start afresh or continue with what we have and build upon from where we have learned.
Blade: That’s very interesting. But can we assume, then, from the clinical trials that have taken place for an HIV vaccine that they did not succeed in providing the immunity needed for an effective vaccine?
Dieffenbach: So, that’s exactly the problem we have. Back in 2007 we had the first hint that an AIDS vaccine might be possible with a study called RV144. We spent 10 years trying to replicate that, and we just completed that study – a study called HVTN702. And it showed no efficacy. So, that was a big disappointment to us. But in the meantime, we had pushed forward with the J&J vaccine and are pretty far along. We’ll see what happens. We should know in the next several months whether the N26 version of an AIDS vaccine, and HIV vaccine works or not. We’re very close to an answer.
Blade: So, the human trials are ongoing.
Dieffenbach: Oh, again – the study in young women in sub-Sahara Africa is fully enrolled. The men’s study will be fully enrolled in September. So, we have fought through the coronavirus epidemic to maintain, to nurse these trials along to make sure with the $100 million or so we’ve invested, that we didn’t want them to go down the drain literally because we lost too many people for follow-up. So, this was a herculean effort that has gone on all the time trying to do the vaccine studies for coronavirus disease, which we were also incredibly successful in.
Blade: Can we assume all of the people participating in the studies were HIV negative?
Dieffenbach: Yes, they’re HIV negative. They are people who are at risk. And also, in South America, for example, the major countries we’re in are Peru and Brazil. And they’ve had a strong research culture with us, going back more than a decade. For example, both of those countries played big roles in our studies of pre-exposure prophylaxis. A study called I-PREX that demonstrated that in men who have sex with men that [a PrEP drug] works well to prevent HIV acquisition in seronegative men who have sex with men.
So, we’ve been there. This is a really good setup for the countries, for the citizens that are in those countries that want to avail themselves to the research that has benefited everybody.
Blade: Among those who are participating in these ongoing AIDS vaccine trials, can we assume they cannot be taking the PrEP anti-retroviral drugs that have been shown to be highly effective in preventing HIV infection?
Dieffenbach: So, what we’ve done is we – everything is by conversation. So, when somebody who is interested in the study comes in, we talk to them. What is your chief interest in being in this study? And a lot of people want to be in the study because then they can access PrEP. They want to make it easier to get a hold of pre-exposure prophylaxis. They feel that is the best way that they can protect themselves.
So, in that situation, what we do is we take those people and link them to PrEP services where they can easily get PrEP in their community. So, first it’s taking care of those people. Then there are people who really have no interest in PrEP. And we actually counsel them every time they come in for a study. Are you sure you don’t want to access PrEP? And those are the people we then say, if you’re not interested in PrEP, what do you think about participating in a vaccine trial?
Because they’re the ones who have the most freedom of thought. They don’t have an opinion about the vaccine or about PrEP. So, those are the people we’ve been focusing on and enrolling. So, we’ve been very careful to make sure that if people wanted PrEP they not only have access, but they didn’t feel like somehow having to trade something in order to get it. The freedom to join a study should be a free choice. And it shouldn’t be a coercive thing to get PrEP. So, we just took that off the table and said if you’re truly interested in PrEP we can get you PrEP and make sure that was available.
Blade: So, in that case, if they choose PrEP they would not be in the vaccine trial?
Dieffenbach: You know, it’s interesting that you ask it in that way. Because you have relationships with your community, many of the investigators have reported that people will say, you know I tried PrEP and it wasn’t for me. It made me gaseous. It upset my stomach. I wasn’t myself. I tried it. I couldn’t make it work for me. I want to stop PrEP. Am I still eligible for the [vaccine] study? And the answer is of course. Many people are very happy on PrEP and they come in for visits occasionally and say this is working for me and just have the relationship with the doctors there, so it works. So, again, it’s about maintaining contact with your communities.
Blade: Can you tell a little about what happens next after people become part of an HIV vaccine trial. Do you have to keep in touch with these people, and do they have to get an HIV test periodically?
Dieffenbach: Exactly. So, the vaccine consists of a series of injections. It’s a mixture of vector systems that delivers a series of encoded HIV genes that are specifically designed to induce very broad immunity. There’s a whole computer-based process to design those components of the vaccine to make sure that it has sequence similarities with all the different versions of HIV circulating in the globe. And then at the end there is a protein boost. And we carry this out.
So, about every three to four months people come in. They get a shot. They fill out questionnaires. They give a blood sample. And they’re tested for HIV and are given a boost or a placebo. And they stay in touch with the clinic. They come in and out of the clinic. And the retention is quite high in these situations because people really like having the attention of the clinic available to them. It’s part of the community.
Blade: So, they go to a clinic for all of this?
Dieffenbach: It’s a research clinic. It’s not like a state-run health clinic. It’s a research clinic. Clinic is just a term for where people are seen.
Blade: Are any of these AIDS vaccine trials that are going on taking place in the United States?
Dieffenbach: Yes. So, the study is called Mosaico. And it’s HVTN706. And we have sites throughout the United States as well as South America. But that study is limited to men who have sex with men – the one in the United States.
Blade: Is it broader than just men who have sex with men in other countries?
Dieffenbach: No, so we decided to really focus on specific at-risk populations. So, in the Americas we chose to focus on men who have sex with men and transgender individuals. And sub-Saharan Africa we focused on young women because that is the target of the study population. So, 705 is all women in sub-Saharan Africa. And in the Americas in North and South America it is all men who have sex with men and transgender individuals.
Blade: Can we assume that the researchers that are doing these studies have a sensitivity of LGBTQ people? Is there still an issue where people worry about being outed as being gay or transgender?
Dieffenbach: So, many of the sites that we work with have been part of our system for over 20 years. And so, they are trusted members of the LGBTQ community within their cities and states. And ‘states’ is a literal term where it’s a state in Colombia or Peru or Brazil. And so, it is part of the fabric of the gay community in these places. Just like in San Francisco the San Francisco health clinic and the DCF clinics are part and parcel of everything the community does there.
And so, the lead physician in San Francisco is Susan Buchbinder. She has been a leader in health in this population for over 25 years or actually closer to 30 years at this point. We’re all getting old. Do you know that? So, we have been at this a very long time. And really have tried to build structures that are durable and therefore are reliable to the community. And that’s where we go back to the same groups time after time.
Blade: Have the locations of the vaccine testing sites been released publicly?
Dieffenbach: Yes, all of that is publicly available on clinicaltrials.gov. If you go into clinicaltrials.gov and search HVTN705 or HVTN706 you will get a version of the protocol, all the times it’s been modified, where we are – the protocol. All of that is public knowledge and available to you. HVTN705 is the women’s study. HVTN706 is the men’s study.
Blade: Is there a timeframe for when these latest vaccine studies might be completed?
Dieffenbach: I think within the next several months. We will get an answer out of the women’s study and then the men’s study is probably a year away. We were slowed a little bit because of COVID. We actually had to pause enrollment for several months. But we’re back on track.
Blade: Isn’t there a parallel research effort for an HIV/AIDS cure?
Dieffenbach: Yes, we have a very large program in cure research. It is a lot earlier in the discovery process and so it’s still very ‘researchy.’ And we have a very large program called the Martin Delany Collaboratories for Cure Research. Martin Delany was an activist who really pushed NIH in so many wonderful ways to really take the need for a cure seriously. His argument was a cure is the next logical step after effective anti-retroviral therapy. You cannot stop with one pill once a day. You’ve got to keep going. And he was pretty persistent. And unfortunately, he died several years go and we just thought the best way to honor him, and his memory was to name a program after him.
Editor’s note: Next week, in the second and final installment of his interview with the Blade, Dr. Dieffenbach discusses the progress in research and studies into an HIV/AIDS cure and explains from a scientific standpoint why an HIV vaccine is taking longer to develop than a COVID vaccine.
The White House
Four states to ignore new Title IX rules protecting transgender students
Biden administration last Friday released final regulations
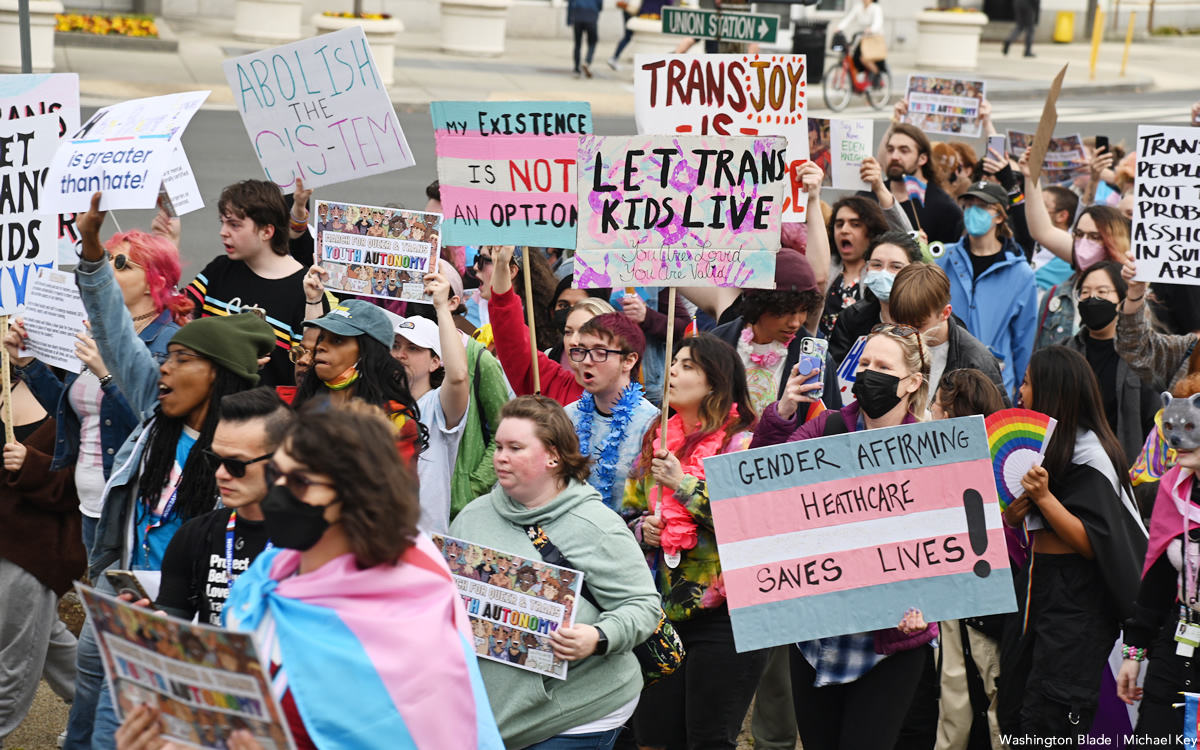
BY ERIN REED | Last Friday, the Biden administration released its final Title IX rules, which include protections for LGBTQ students by clarifying that Title IX forbids discrimination based on sexual orientation and gender identity.
The rule change could have a significant impact as it would supersede bathroom bans and other discriminatory policies that have become increasingly common in Republican states within the U.S.
As of Thursday morning, however, officials in at least four states — Oklahoma, Louisiana, Florida, and South Carolina — have directed schools to ignore the regulations, potentially setting up a federal showdown that may ultimately end up in a protracted court battle in the lead-up to the 2024 elections.
Louisiana State Superintendent of Education Cade Brumley was the first to respond, decrying the fact that the new Title IX regulations could block teachers and other students from exercising what has been dubbed by some a “right to bully” transgender students by using their old names and pronouns intentionally.
Asserting that Title IX law does not protect trans and queer students, Brumley states that schools “should not alter policies or procedures at this time.” Critically, several courts have ruled that trans and queer students are protected by Title IX, including the 4th U.S. Circuit Court of Appeals in a recent case in West Virginia.
In South Carolina, Schools Supt. Ellen Weaver wrote in a letter that providing protections for trans and LGBTQ students under Title IX “would rescind 50 years of progress and equality of opportunity by putting girls and women at a disadvantage in the educational arena,” apparently leaving trans kids out of her definition of those who deserve progress and equality of opportunity.
She then directed schools to ignore the new directive while waiting for court challenges. While South Carolina does not have a bathroom ban or statewide “Don’t Say Gay or Trans” law, such bills continue to be proposed in the state.
Responding to the South Carolina letter, Chase Glenn of Alliance For Full Acceptance stated, “While Supt. Weaver may not personally support the rights of LGBTQ+ students, she has the responsibility as the top school leader in our state to ensure that all students have equal rights and protections, and a safe place to learn and be themselves. The flagrant disregard shown for the Title IX rule tells me that our superintendent unfortunately does not have the best interests of all students in mind.”
Florida Education Commissioner Manny Diaz also joined in instructing schools not to implement Title IX regulations. In a letter issued to area schools, Diaz stated that the new Title IX regulations were tantamount to “gaslighting the country into believing that biological sex no longer has any meaning.”
Governor Ron DeSantis approved of the letter and stated that Florida “will not comply.” Florida has notably been the site of some of the most viciously anti-queer and anti-trans legislation in recent history, including a “Don’t Say Gay or Trans” law that was used to force a trans female teacher to go by “Mr.”
State Education Supt. Ryan Walters of Oklahoma was the latest to echo similar sentiments. Walters has recently appointed the right-wing media figure Chaya Raichik of Libs of TikTok to an advisory role “to improve school safety,” and notably, Raichik has posed proudly with papers accusing her of instigating bomb threats with her incendiary posts about LGBTQ people in classrooms.
The Title IX policies have been universally applauded by large LGBTQ rights organizations in the U.S. Lambda Legal, a key figure in fighting anti-LGBTQ legislation nationwide, said that the regulations “clearly cover LGBTQ+ students, as well as survivors and pregnant and parenting students across race and gender identity.” The Human Rights Campaign also praised the rule, stating, “rule will be life-changing for so many LGBTQ+ youth and help ensure LGBTQ+ students can receive the same educational experience as their peers: Going to dances, safely using the restroom, and writing stories that tell the truth about their own lives.”
The rule is slated to go into effect Aug. 1, pending any legal challenges.
****************************************************************************

Erin Reed is a transgender woman (she/her pronouns) and researcher who tracks anti-LGBTQ+ legislation around the world and helps people become better advocates for their queer family, friends, colleagues, and community. Reed also is a social media consultant and public speaker.
******************************************************************************************
The preceding article was first published at Erin In The Morning and is republished with permission.
Pennsylvania
Malcolm Kenyatta could become the first LGBTQ statewide elected official in Pa.
State lawmaker a prominent Biden-Harris 2024 reelection campaign surrogate

Following his win in the Democratic primary contest on Wednesday, Pennsylvania state Rep. Malcolm Kenyatta, who is running for auditor general, is positioned to potentially become the first openly LGBTQ elected official serving the commonwealth.
In a statement celebrating his victory, LGBTQ+ Victory Fund President Annise Parker said, “Pennsylvanians trust Malcolm Kenyatta to be their watchdog as auditor general because that’s exactly what he’s been as a legislator.”
“LGBTQ+ Victory Fund is all in for Malcolm, because we know he has the experience to win this race and carry on his fight for students, seniors and workers as Pennsylvania’s auditor general,” she said.
Parker added, “LGBTQ+ Americans are severely underrepresented in public office and the numbers are even worse for Black LGBTQ+ representation. I look forward to doing everything I can to mobilize LGBTQ+ Pennsylvanians and our allies to get out and vote for Malcolm this November so we can make history.”
In April 2023, Kenyatta was appointed by the White House to serve as director of the Presidential Advisory Commission on Advancing Educational Equity, Excellence and Economic Opportunity for Black Americans.
He has been an active surrogate in the Biden-Harris 2024 reelection campaign.
The White House
White House debuts action plan targeting pollutants in drinking water
Same-sex couples face higher risk from environmental hazards

Headlining an Earth Day event in Northern Virginia’s Prince William Forest on Monday, President Joe Biden announced the disbursement of $7 billion in new grants for solar projects and warned of his Republican opponent’s plans to roll back the progress his administration has made toward addressing the harms of climate change.
The administration has led more than 500 programs geared toward communities most impacted by health and safety hazards like pollution and extreme weather events.
In a statement to the Washington Blade on Wednesday, Brenda Mallory, chair of the White House Council on Environmental Quality, said, “President Biden is leading the most ambitious climate, conservation, and environmental justice agenda in history — and that means working toward a future where all people can breathe clean air, drink clean water, and live in a healthy community.”
“This Earth Week, the Biden-Harris Administration announced $7 billion in solar energy projects for over 900,000 households in disadvantaged communities while creating hundreds of thousands of clean energy jobs, which are being made more accessible by the American Climate Corps,” she said. “President Biden is delivering on his promise to help protect all communities from the impacts of climate change — including the LGBTQI+ community — and that we leave no community behind as we build an equitable and inclusive clean energy economy for all.”
Recent milestones in the administration’s climate policies include the U.S. Environmental Protection Agency’s issuance on April 10 of legally enforceable standard for detecting and treating drinking water contaminated with polyfluoroalkyl substances.
“This rule sets health safeguards and will require public water systems to monitor and reduce the levels of PFAS in our nation’s drinking water, and notify the public of any exceedances of those levels,” according to a White House fact sheet. “The rule sets drinking water limits for five individual PFAS, including the most frequently found PFOA and PFOS.”
The move is expected to protect 100 million Americans from exposure to the “forever chemicals,” which have been linked to severe health problems including cancers, liver and heart damage, and developmental impacts in children.
An interactive dashboard from the United States Geological Survey shows the concentrations of polyfluoroalkyl substances in tapwater are highest in urban areas with dense populations, including cities like New York and Los Angeles.
During Biden’s tenure, the federal government has launched more than 500 programs that are geared toward investing in the communities most impacted by climate change, whether the harms may arise from chemical pollutants, extreme weather events, or other causes.
New research by the Williams Institute at the UCLA School of Law found that because LGBTQ Americans are likelier to live in coastal areas and densely populated cities, households with same-sex couples are likelier to experience the adverse effects of climate change.
The report notes that previous research, including a study that used “national Census data on same-sex households by census tract combined with data on hazardous air pollutants (HAPs) from the National Air Toxics Assessment” to model “the relationship between same-sex households and risk of cancer and respiratory illness” found “that higher prevalence of same-sex households is associated with higher risks for these diseases.”
“Climate change action plans at federal, state, and local levels, including disaster preparedness, response, and recovery plans, must be inclusive and address the specific needs and vulnerabilities facing LGBT people,” the Williams Institute wrote.
With respect to polyfluoroalkyl substances, the EPA’s adoption of new standards follows other federal actions undertaken during the Biden-Harris administration to protect firefighters and healthcare workers, test for and clean up pollution, and phase out or reduce use of the chemicals in fire suppressants, food packaging, and federal procurement.
-

 District of Columbia3 days ago
District of Columbia3 days agoCatching up with the asexuals and aromantics of D.C.
-

 South America3 days ago
South America3 days agoArgentina government dismisses transgender public sector employees
-

 Maine4 days ago
Maine4 days agoMaine governor signs transgender, abortion sanctuary bill into law
-

 District of Columbia4 days ago
District of Columbia4 days agoBowser budget proposal calls for $5.25 million for 2025 World Pride