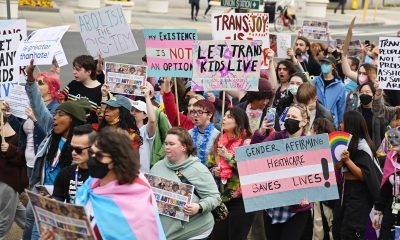Health
FDA guidance eases blood donation restrictions for gay, bi men
Sen. Tammy Baldwin, Congressional LGBTQ+ Equality Caucus, LGBTQ groups celebrate new FDA proposal

The U.S. Food and Drug Administration (FDA) introduced a proposed change to its blood donation guidelines on Friday that would ease restrictions for gay and bisexual men.
The FDA notes the proposal, news of which was first reported in November, would bring U.S. policies in alignment with those in place in countries like the U.K. and Canada. The agency is expected to formally adopt the new guidelines after a public comment period.
The move follows criticism from LGBTQ groups and organizations like the American Medical Association (AMA) that have long argued the current policy is homophobic and based on an outdated understanding of the risks associated with blood donation by men who have sex with men.
As the AMA wrote of the current policy: “a man who has protected sex with another man in the three months prior to a blood donation cannot be a donor, but a man or woman who has unprotected sex with multiple partners of the opposite sex over the same time period remains eligible.”
The FDA’s proposal would lift the mandatory three-month deferral period for some men who have sex with men and instead use a “gender-inclusive, individual risk-based questions relevant to HIV risk.”
Potential donors would be asked for information about their sexual history over the past three months. Respondents who indicate they have had sex with one or more new sexual partners would then be asked whether they have had anal sex during this period. Those who answer “yes” would be deferred from blood donation.
Axios noted that as of this morning, about 20 percent of the country’s community blood centers have a one-day supply or less, while the FDA’s broadened eligibility criteria would increase the annual blood supply by two to four percent, citing data from America’s Blood Centers’ daily tracker and the Williams Institute.
U.S. Sen. Tammy Baldwin (D-Wis.) issued a statement celebrating the FDA’s proposal. “As I have long advocated for, this blood donation policy takes a step forward and is better rooted in the most up-to-date science with a focus on individual risk factors, not outdated stigmas that effectively ban gay and bisexual men,” she said.
Baldwin has repeatedly urged the agency to revisit its blood donation policy over the years, including by corralling support from other members of Congress to cosign letters to the FDA in 2014 and 2016, raising the issue again in 2020 as the COVID-19 pandemic exacerbated shortages in the blood supply.
The Congressional LGBTQ+ Equality Caucus also acknowledged the move in a statement by its chair, Rep. Mark Pocan (D-Wis.): “I am glad the FDA is finally moving toward an individual risk-based assessment model, but recognize, based on existing reporting, that many LGBTQI+ people may still be barred from donating,” he said. “I look forward to taking a closer look at the proposed guidelines once they are published and working with the FDA to ensure that any unnecessary barriers are removed.”
Several LGBTQ groups also issued statements celebrating the FDA’s new guidance.
“These changes are 40-plus years in the making, and are a tremendous leap forward toward elevating science over stigma,” said GLAAD President Sarah Kate Ellis. “GLAAD and leading medical experts have long been advocating for guidelines that see and treat LGBTQ people the same as any other person, including as potential donors who want to help others.”
“This new policy removes a decades-long barrier for many in our community – and there is more to do to ensure gay, bisexual and transgender people are no longer unfairly stigmatized when they try to donate blood,” Human Rights Campaign President Kelley Robinson said. “The assessment criteria have flaws, focusing excessively, for instance, on the number of partners a potential donor has instead of just on new partners,” she added.
Carl Schmid, executive director of the HIV+ Hepatitis Policy Institute, said: “While this long-overdue change is being made based on the science and the facts, which have been clear for years, it is the result of the leadership of the Biden administration that continues to tear down discriminatory government policies.”
Health
UNAIDS to commemorate Zero Discrimination Day’s 10th anniversary
UN agency urges global action to protect human rights

As the world marks the 10th anniversary of Zero Discrimination Day; UNAIDS is sounding the alarm on the increasing threats to human rights, calling for renewed efforts to protect the rights of all individuals as a fundamental step towards ensuring health for everyone.
Established by UNAIDS a decade ago, Zero Discrimination Day aims to promote equality and fairness regardless of gender, age, sexuality, ethnicity or HIV status. The progress achieved over the past years is now in jeopardy, however, due to rising attacks on the rights of women, LGBTQ people and other marginalized communities.
UNAIDS Executive Director Winnie Byanyima emphasized the critical link between protecting human rights and safeguarding public health.
“The attacks on rights are a threat to freedom and democracy and are harmful to health,” she said in a press release. “Stigma and discrimination obstruct HIV prevention, testing, treatment and care and hold back progress towards ending AIDS by 2030. It is only by protecting everyone’s rights that we can protect everyone’s health.”
Despite challenges, there has been notable progress.
At the onset of the AIDS pandemic more than 40 years ago, two-thirds of countries criminalized consensual same-sex sexual relations. They are now decriminalized in two-thirds of countries. An additional 38 countries around the world have pledged to end HIV-related stigma and discrimination, contributing to positive changes that include 50 million more girls attending school compared to 2015.
To sustain and enhance these advancements; UNAIDS urges global support for women’s rights movements, LGBTQ rights, racial justice, economic justice, climate justice and peace initiatives. By standing with communities advocating for their rights, the U.N. aims to reinforce the collective effort towards a more inclusive and equitable world.
Zero Discrimination Day is observed on March 1.
Events and activities that will take place around the world throughout the month will serve as reminders of the essential lesson and call to action: Protecting everyone’s health is synonymous with protecting everyone’s rights.
“Through upholding rights for all, we will be able to achieve the Sustainable Development Goals and secure a safer, fairer, kinder and happier world — for everyone,” said Byanyima.
Health
New CDC report finds transgender women at higher risk for HIV
More than 1,600 people in seven cities surveyed

The Centers for Disease Control and Prevention issued a new study report this week that revealed that restricted by employment and housing discrimination and lack of access to needed gender-affirming healthcare for transgender women increasing the risk of contracting HIV.
Researchers reviewed data from a 2019-2020 survey, the National HIV Behavioral Surveillance Among Transgender Women, which found that the demographics of HIV/AIDS have been disproportionally high, especially among Black and Latina trans women, who had experienced employment and housing discrimination coupled with lack of access to gender-affirming healthcare.
The Jan. 25 Morbidity and Mortality Weekly Report was based on data studies of more than 1,600 trans women in seven major urban locales. Participants from Atlanta, Los Angeles, New Orleans, New York, Philadelphia, San Francisco and Seattle were chosen by referrals from people and community-based organizations who knew or were part of the local population of trans women.
The study’s researchers noted: “Employment discrimination occurs at the overlapping nexus of poverty, homelessness, incarceration, health insurance, disability, food insecurity and survival sex work. These issues are interconnected.”
The study stated that trans women’s inability to access quality healthcare, including gender-affirming treatment or access to PrEP, and can expose them to potential incarceration as many turn to “survival sex work” and violence, which increases the risk of contracting HIV.
The study’s author’s pointed out: “When economically marginalized transgender women are refused employment, this refusal cyclically contributes to economic hardships. This analysis …demonstrates the importance of transgender women working and living with dignity and without fear of unfair treatment.”
Health
A Whole New Perspective on Well-Being
The Mather’s team recognizes that everyone’s wellness journey is completely unique to their life experiences and influences.

It’s easy to spot the distinctive, elegant silhouette of The Mather, a Life Plan Community for those 62+ opening this spring in Tysons, Virginia. What is not apparent to the naked eye is The Mather’s unique wellness philosophy, which is literally built into the community.
The Mather’s team recognizes that everyone’s wellness journey is completely unique to their life experiences and influences.
Nature is one of the important factors that contribute to well-being. So The Mather is incorporating biophilic design—a design approach to facilitate access to nature or things that replicate natural patterns. This can include interior spaces with sightlines to a garden, choosing natural wood and stone as interior materials, or incorporating fragrant flowers and plants indoors to spark memories and provide tactile opportunities such as gardening.

“Providing biophilic design within interior settings connects residents to the natural world,” says Mary Leary, CEO and President of Mather, the organization behind The Mather. “Research shows that a connection to nature provides positive benefits to mental states and overall well-being. At The Mather, biophilic design is the intersection of buildings and programs with nature in an urban setting.”
“The Mather is attracting a diverse group of older adults,” says Mary. “As a result, we aim to incorporate wellness practices from around the world, including Wyda movement theory of the Celtic Druids, which helps people achieve harmony with nature and contentment through mindfulness.” This holistic regenerative approach is similar to Qi Gong and yoga, while born in a different part of the world. Mather Institute has a special focus on mindfulness to support older adults’ practice of present moment awareness, which can lead to increased overall well-being, compassion, and joy.
A very different example of a wellness offering at The Mather is the Gharieni Welnamis spa wave bed, which uses computer-controlled vibrational therapy and audio frequencies to train the brain to relax. “The bed increases mindfulness, concentration, and creativity—all of which support our mission of creating Ways to Age Well,SM” says Mary.
These and other personalized ways to wellness will ensure that residents of The Mather can choose from seemingly countless ways to focus on their well-being. In other words, the sky’s the limit!
-

 State Department4 days ago
State Department4 days agoState Department releases annual human rights report
-

 South America2 days ago
South America2 days agoArgentina government dismisses transgender public sector employees
-

 District of Columbia2 days ago
District of Columbia2 days agoCatching up with the asexuals and aromantics of D.C.
-

 Politics5 days ago
Politics5 days agoSmithsonian staff concerned about future of LGBTQ programming amid GOP scrutiny