Health
GWU participates in ‘promising’ HIV vaccine study
Medical school researchers hopeful new approach will prevent infection
D.C.’s George Washington University School of Medicine and Health Sciences is one of four sites across the country in which a preliminary component of an experimental HIV vaccine is being given to volunteer participants in a study aimed at reversing years of failed attempts to develop an effective HIV vaccine by pursuing what study sponsors say is a new, promising approach.
The study, which involves 56 healthy, HIV-negative volunteer participants, is being conducted by the nonprofit scientific research organization known as IAVI and the biotechnology company Moderna, which developed one of the coronavirus vaccines now being used throughout the world.
In a Jan. 27 joint statement, IAVI and Moderna said their study is part of a Phase 1 trial designed to test newly developed experimental HIV vaccine antigens to determine if they will lead to the development of an effective HIV vaccine.
According to scientific literature, antigens are substances such as bacteria, viruses, and chemicals that induce the body to release antibodies that fight off infections. The statement by IAVI and Moderna says a vaccine technology developed by Moderna to use another component of the human body called messenger RNA or mRNA to strengthen a potential vaccine’s ability to fight off infection by HIV is also a part of this vaccine study.
“We are tremendously excited to be advancing this new direction in HIV vaccine design with Moderna’s mRNA platform,” Mark Feinberg, president and CEO of IAVI, says in the statement. “The search for an HIV vaccine has been long and challenging and having new tools in terms of immunogens and platforms could be the key to making rapid progress toward an urgently needed, effective HIV vaccine,” he says in the statement.
The statement says that scientific teams at IAVI and the biotechnology firm Scripps Research helped to develop the HIV vaccine antigens being tested in the trials taking place at the GW School of Medicine and Health Sciences and at locations in Atlanta, Ga., Seattle, Wash., and San Antonio, Tex.
It says the trial involving the 56 volunteer participants — who are divided among the four sites — began on Jan. 27 and is being funded by the Bill & Melinda Gates Foundation.
Among those calling the IAVI-Moderna trial an important step in HIV vaccine development is Carl Dieffenbach, director of the Division of AIDS at the National Institute of Allergies and Infectious Diseases (NIAID), which is part of the U.S. National Institutes of Health.
“This is a variation of a theme,” Dieffenbach told the Washington Blade. “IAVI in collaboration with NIH did a version of this study already with a protein form of this immunogen,” Dieffenbach said. He said that study worked out well and was published in a scientific journal.
“What’s unique about this latest study is they’re using RNA to deliver the vaccine rather than a protein,” said Dieffenbach. “So, this is an important step for us in the vaccine field, that they can now compare the protein to the RNA.”
Dieffenbach said the IAVI-Moderna trial is taking place after two other recently completed HIV vaccine studies involving human trials that NIAID was involved in resulted in findings that the two experimental HIV vaccines were ineffective. He said a third HIV vaccine study NIAID is involved in that is taking place in the U.S. and South America is expected to be completed in about a year.
The ongoing study in the Americas involves men who have sex with men and transgender individuals as those participating in that vaccine trial, he said.
Dieffenbach said in addition to the vaccine studies, NIAID is monitoring at least two studies of medication aimed at curing HIV. One of the studies was conducted by HIV researcher Dr. Timothy Schacker, who serves as Vice Dean for research at the University of Minnesota Medical School.
Schacker arranged for human trials of people who are HIV positive and taking standard anti-retroviral HIV medication to be given an experimental HIV cure medication developed by the biotechnology company ImmunityBio called Anktiva, according to a Jan. 31 statement released by ImmunityBio.
The statement says the trials showed promising results in the ability of Anktiva to induce the immune system of HIV-positive patients under standard HIV treatment who participated in the study to “kill” the latent or “hidden” HIV in their body that would otherwise reactivate and cause illness if they stopped taking HIV medication.
The goal of the development of Anktiva is to “rid the body of the virus for good and eliminate the need for antiretroviral therapy,” the company’s statement says.
Dieffenbach said his office was also monitoring an HIV cure study being conducted by the Rockville, Md., based genetic engineering company called American Gene Technologies. The company is conducting a human trial for a therapeutic treatment it has developed that’s intended to enable the immune system of HIV-positive people to permanently eliminate HIV from their bodies. The company has said it was hopeful that early results of the effectiveness of the treatment would become available this year.
Monkeypox
US contributes more than $90 million to fight mpox outbreak in Africa
WHO and Africa CDC has declared a public health emergency
The U.S. has contributed more than $90 million to the fight against the mpox outbreak in Africa.
The U.S. Agency for International Development on Tuesday in a press release announced “up to an additional” $35 million “in emergency health assistance to bolster response efforts for the clade I mpox outbreak in Central and Eastern Africa, pending congressional notification.” The press release notes the Biden-Harris administration previously pledged more than $55 million to fight the outbreak in Congo and other African countries.
“The additional assistance announced today will enable USAID to continue working closely with affected countries, as well as regional and global health partners, to expand support and reduce the impact of this outbreak as it continues to evolve,” it reads. “USAID support includes assistance with surveillance, diagnostics, risk communication and community engagement, infection prevention and control, case management, and vaccination planning and coordination.”
The World Health Organization and the Africa Centers for Disease Control and Prevention last week declared the outbreak a public health emergency.
The Washington Blade last week reported there are more than 17,000 suspected mpox cases across in Congo, Uganda, Kenya, Rwanda, and other African countries. The outbreak has claimed more than 500 lives, mostly in Congo.
Health
Mpox outbreak in Africa declared global health emergency
ONE: 10 million vaccine doses needed on the continent

Medical facilities that provide treatment to gay and bisexual men in some East African countries are already collaborating with them to prevent the spread of a new wave of mpox cases after the World Health Organization on Wednesday declared a global health emergency.
The collaboration, both in Uganda and Kenya, comes amid WHO’s latest report released on Aug. 12, which reveals that nine out of every 10 reported mpox cases are men with sex as the most common cause of infection.
The global mpox outbreak report — based on data that national authorities collected between January 2022 and June of this year — notes 87,189 of the 90,410 reported cases were men. Ninety-six percent of whom were infected through sex.
Sexual contact as the leading mode of transmission accounted for 19,102 of 22,802 cases, followed by non-sexual person-to-person contact. Genital rash was the most common symptom, followed by fever and systemic rash.
The WHO report states the pattern of mpox virus transmission has persisted over the last six months, with 97 percent of new cases reporting sexual contact through oral, vaginal, or anal sex with infected people.
“Sexual transmission has been recorded in the Democratic Republic of Congo among sex workers and men who have sex with men,” the report reads. “Among cases exposed through sexual contact in the Democratic Republic of the Congo, some individuals present only with genital lesions, rather than the more typical extensive rash associated with the virus.”
The growing mpox cases, which are now more than 2,800 reported cases in at least 13 African countries that include Kenya, Uganda, Rwanda, and prompted the Africa Centers for Disease Control and Prevention this week to declare the disease a public health emergency for resource mobilization on the continent to tackle it.
“Africa has long been on the frontlines in the fight against infectious diseases, often with limited resources,” said Africa CDC Director General Jean Kaseya. “The battle against Mpox demands a global response. We need your support, expertise, and solidarity. The world cannot afford to turn a blind eye to this crisis.”
The disease has so far claimed more than 500 lives, mostly in Congo, even as the Africa CDC notes suspected mpox cases across the continent have surged past 17,000, compared to 7,146 cases in 2022 and 14,957 cases last year.
“This is just the tip of the iceberg when we consider the many weaknesses in surveillance, laboratory testing, and contact tracing,” Kaseya said.
WHO, led by Director General Tedros Adhanom Ghebreyesus, also followed the Africa CDC’s move by declaring the mpox outbreak a public health emergency of international concern.
The latest WHO report reveals that men, including those who identify as gay and bisexual, constitute most mpox cases in Kenya and Uganda. The two countries have recorded their first cases, and has put queer rights organizations and health care centers that treat the LGBTQ community on high alert.
The Uganda Minority Shelters Consortium, for example, confirmed to the Washington Blade that the collaboration with health service providers to prevent the spread of mpox among gay and bisexual men is “nascent and uneven.”
“While some community-led health service providers such as Ark Wellness Clinic, Children of the Sun Clinic, Ice Breakers Uganda Clinic, and Happy Family Youth Clinic, have demonstrated commendable efforts, widespread collaboration on mpox prevention remains a significant gap,” UMSC Coordinator John Grace stated. “This is particularly evident when compared to the response to the previous Red Eyes outbreak within the LGBT community.”
Grace noted that as of Wednesday, there were no known queer-friendly health service providers to offer mpox vaccinations to men who have sex with men. He called for health care centers to provide inclusive services and a more coordinated approach.
Although Grace pointed out the fear of discrimination — and particularly Uganda’s Anti-Homosexuality Act — remains a big barrier to mpox prevention through testing, vaccination, and treatment among queer people, he confirmed no mpox cases have been reported among the LGBTQ community.
Uganda so far has reported two mpox cases — refugees who had travelled from Congo.
“We are for the most part encouraging safer sex practices even after potential future vaccinations are conducted as it can also be spread through bodily fluids like saliva and sweat,” Grace said.
Grace also noted that raising awareness about mpox among the queer community and seeking treatment when infected remains a challenge due to the historical and ongoing homophobic stigma and that more comprehensive and reliable advocacy is needed. He said Grindr and other digital platforms have been crucial in raising awareness.
The declarations of mpox as a global health emergency have already attracted demand for global leaders to support African countries to swiftly obtain the necessary vaccines and diagnostics.
“History shows we must act quickly and decisively when a public health emergency strikes. The current Mpox outbreak in Africa is one such emergency,” said ONE Global Health Senior Policy Director Jenny Ottenhoff.
ONE is a global, nonpartisan organization that advocates for the investments needed to create economic opportunities and healthier lives in Africa.
Ottenhoff warned failure to support the African countries with medical supplies needed to tackle mpox would leave the continent defenseless against the virus.
To ensure that African countries are adequately supported, ONE wants governments and pharmaceutical companies to urgently increase the provision of mpox vaccines so that the most affected African countries have affordable access to them. It also notes 10 million vaccine doses are currently needed to control the mpox outbreak in Africa, yet the continent has only 200,000 doses.
The Blade has reached out to Ishtar MSM, a community-based healthcare center in Nairobi, Kenya, that offers to service to gay and bisexual men, about their response to the mpox outbreak.
Health
White House urged to expand PrEP coverage for injectable form
HIV/AIDS service organizations made call on Wednesday
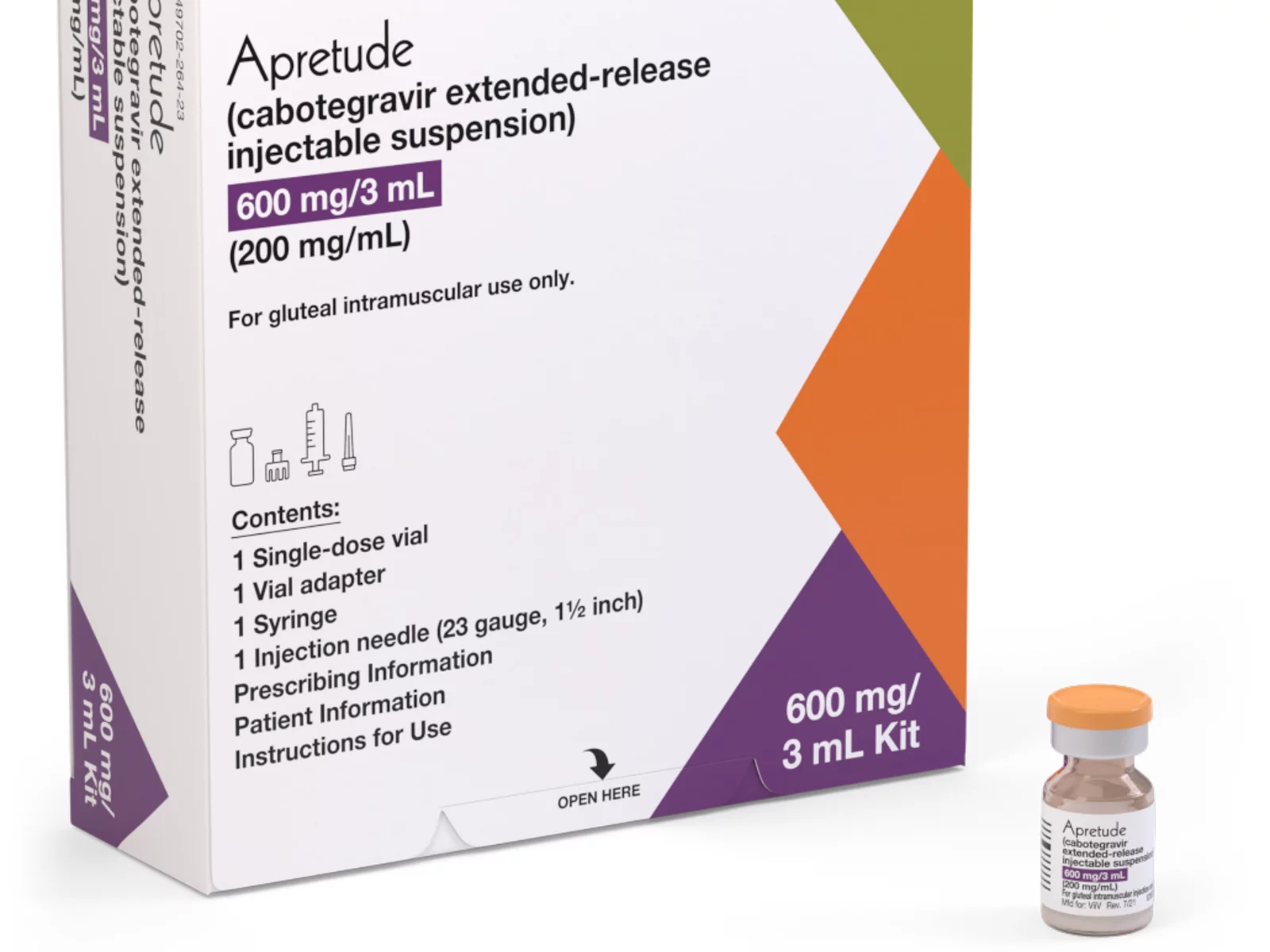
A coalition of 63 organizations dedicated to ending HIV called on the Biden-Harris administration on Wednesday to require insurers to cover long-acting pre-exposure prophylaxis (PrEP) without cost-sharing.
In a letter to Chiquita Brooks-LaSure, administrator of the Centers for Medicare and Medicaid Services, the groups emphasized the need for broad and equitable access to PrEP free of insurance barriers.
Long-acting PrEP is an injectable form of PrEP that’s effective over a long period of time. The FDA approved Apretude (cabotegravir extended-release injectable suspension) as the first and only long-acting injectable PrEP in late 2021. It’s intended for adults and adolescents weighing at least 77 lbs. who are at risk for HIV through sex.
The U.S. Preventive Services Task Force updated its recommendation for PrEP on Aug. 22, 2023, to include new medications such as the first long-acting PrEP drug. The coalition wants CMS to issue guidance requiring insurers to cover all forms of PrEP, including current and future FDA-approved drugs.
“Long-acting PrEP can be the answer to low PrEP uptake, particularly in communities not using PrEP today,” said Carl Schmid, executive director of the HIV+Hepatitis Policy Institute. “The Biden administration has an opportunity to ensure that people with private insurance can access PrEP now and into the future, free of any cost-sharing, with properly worded guidance to insurers.”
Currently, only 36 percent of those who could benefit from PrEP are using it. Significant disparities exist among racial and ethnic groups. Black people constitute 39 percent of new HIV diagnoses but only 14 percent of PrEP users, while Latinos represent 31 percent of new diagnoses but only 18 percent of PrEP users. In contrast, white people represent 24 percent of HIV diagnoses but 64 percent of PrEP users.
The groups also want CMS to prohibit insurers from employing prior authorization for PrEP, citing it as a significant barrier to access. Several states, including New York and California, already prohibit prior authorization for PrEP.
Modeling conducted for HIV+Hep, based on clinical trials of a once every 2-month injection, suggests that 87 percent more HIV cases would be averted compared to daily oral PrEP, with $4.25 billion in averted healthcare costs over 10 years.
Despite guidance issued to insurers in July 2021, PrEP users continue to report being charged cost-sharing for both the drug and ancillary services. A recent review of claims data found that 36 percent of PrEP users were charged for their drugs, and even 31 percent of those using generic PrEP faced cost-sharing.
The coalition’s letter follows a more detailed communication sent by HIV+Hepatitis Policy Institute to the Biden administration on July 2.
Signatories to the community letter include Advocates for Youth, AIDS United, Equality California, Fenway Health, Human Rights Campaign, and the National Coalition of STD Directors, among others.
-

 Federal Government2 days ago
Federal Government2 days agoTreasury Department has a gay secretary but LGBTQ staff are under siege
-

 Virginia3 days ago
Virginia3 days agoDefying trends, new LGBTQ center opens in rural Winchester, Va.
-

 District of Columbia2 days ago
District of Columbia2 days agoGay GOP group hosts Ernst, 3 House members — all of whom oppose Equality Act
-

 District of Columbia2 days ago
District of Columbia2 days agoD.C. police seek public’s help in July 5 murder of trans woman










