The White House
U.S. orders 2.5 million more monkeypox vaccine doses
CDC has reported roughly 350 cases
The U.S. Department of Health and Human Services announced Friday that it has ordered an additional 2.5 million doses of Bavarian Nordic’s JYNNEOS, an FDA-licensed vaccine indicated for prevention of smallpox and monkeypox, for use in responding to current or future monkeypox outbreaks and as part of U.S. smallpox preparedness.
Deliveries from this latest order of the Bavarian Nordic‘s Jynneos vaccine will begin arriving at the Strategic National Stockpile (SNS) later this year and will continue through early 2023 HHS said in a statement.
“We are working around-the-clock with public health officials in states and large metro areas to provide them with vaccines and treatments to respond to the current monkeypox outbreak,” said HHS Secretary Xavier Becerra. “This order of additional JYNNEOS vaccine will help us push out more vaccine quickly, knowing that we have more doses on the way in the coming months — and is only possible because of our longstanding investment in smallpox and monkeypox preparedness.”
The order announced today is in addition to the 500,000 doses of government-owned vaccine the company is producing in 2022 for use in the current response to monkeypox in the U.S and brings the total vaccine doses to be delivered in 2022 and 2023 to more than 4 million.
The company will produce these doses in liquid frozen form using vaccine already manufactured in bulk under an existing 10-year contract with the Biomedical Advanced Research and Development Authority, within the HHS Office of the Assistant Secretary for Preparedness and Response; that contract was part of ongoing national preparedness efforts against smallpox.
“The medical countermeasures available to help respond to the current outbreak are the result of years of investment and planning made possible through the ongoing work between HHS and private industry,” said Gary Disbrow, director of the Biomedical Advanced Research and Development Authority. “We are pleased that we have been able to work with our partners at Bavarian Nordic to accelerate delivery of vaccines that can help keep people safe and stem the spread of the virus.”
BARDA supported the development of JYNNEOS, which is approved by the FDA to prevent smallpox and monkeypox. The U.S. government owns enough smallpox vaccine — JYNNEOS and ACAM2000 — to vaccinate millions of Americans, if needed.
As of June 24, ASPR’s SNS held approximately 65,000 doses of JYNNEOS in immediate inventory with delivery of an additional 300,000 doses in the coming days. On June 28, HHS announced that it would immediately make available 56,000 doses and soon after would make available 240,000 additional doses. The SNS also has more than 100 million doses of ACAM2000 which was developed with SNS support and is approved by FDA for use in preventing smallpox. The Centers for Disease Control and Prevention currently has an expanded access Investigational New Drug protocol which allows use of ACAM2000 for monkeypox.
In addition, the SNS has over 1.7 million treatment courses of the smallpox antiviral drug TPOXX, which was developed with BARDA support and can be used to treat individuals with monkeypox under an appropriate regulatory mechanism. CDC currently has an expanded access Investigational New Drug protocol which allows its use for monkeypox.
As of June 29, the CDC has received reports of approximately 350 cases of monkeypox in the U.S., primarily among men who have sex with men.
To learn more about monkeypox, visit cdc.gov/monkeypox.
The White House
Four states to ignore new Title IX rules protecting transgender students
Biden administration last Friday released final regulations
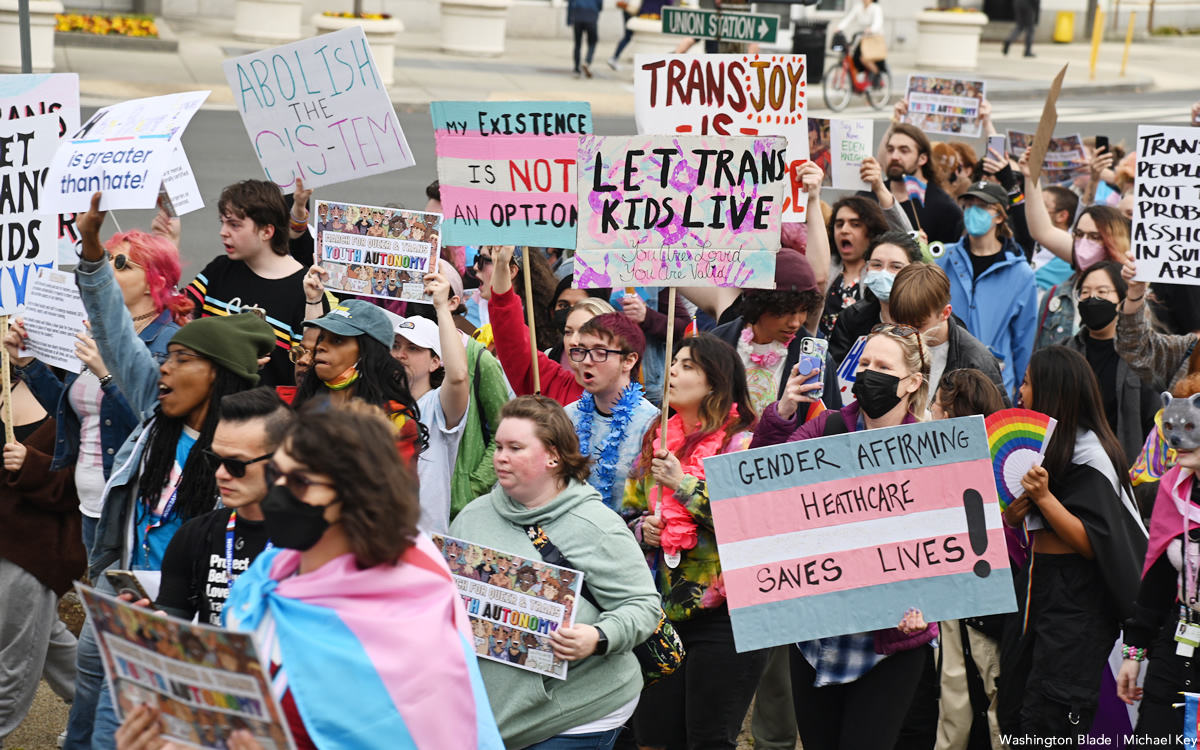
BY ERIN REED | Last Friday, the Biden administration released its final Title IX rules, which include protections for LGBTQ students by clarifying that Title IX forbids discrimination based on sexual orientation and gender identity.
The rule change could have a significant impact as it would supersede bathroom bans and other discriminatory policies that have become increasingly common in Republican states within the U.S.
As of Thursday morning, however, officials in at least four states — Oklahoma, Louisiana, Florida, and South Carolina — have directed schools to ignore the regulations, potentially setting up a federal showdown that may ultimately end up in a protracted court battle in the lead-up to the 2024 elections.
Louisiana State Superintendent of Education Cade Brumley was the first to respond, decrying the fact that the new Title IX regulations could block teachers and other students from exercising what has been dubbed by some a “right to bully” transgender students by using their old names and pronouns intentionally.
Asserting that Title IX law does not protect trans and queer students, Brumley states that schools “should not alter policies or procedures at this time.” Critically, several courts have ruled that trans and queer students are protected by Title IX, including the 4th U.S. Circuit Court of Appeals in a recent case in West Virginia.
In South Carolina, Schools Supt. Ellen Weaver wrote in a letter that providing protections for trans and LGBTQ students under Title IX “would rescind 50 years of progress and equality of opportunity by putting girls and women at a disadvantage in the educational arena,” apparently leaving trans kids out of her definition of those who deserve progress and equality of opportunity.
She then directed schools to ignore the new directive while waiting for court challenges. While South Carolina does not have a bathroom ban or statewide “Don’t Say Gay or Trans” law, such bills continue to be proposed in the state.
Responding to the South Carolina letter, Chase Glenn of Alliance For Full Acceptance stated, “While Supt. Weaver may not personally support the rights of LGBTQ+ students, she has the responsibility as the top school leader in our state to ensure that all students have equal rights and protections, and a safe place to learn and be themselves. The flagrant disregard shown for the Title IX rule tells me that our superintendent unfortunately does not have the best interests of all students in mind.”
Florida Education Commissioner Manny Diaz also joined in instructing schools not to implement Title IX regulations. In a letter issued to area schools, Diaz stated that the new Title IX regulations were tantamount to “gaslighting the country into believing that biological sex no longer has any meaning.”
Governor Ron DeSantis approved of the letter and stated that Florida “will not comply.” Florida has notably been the site of some of the most viciously anti-queer and anti-trans legislation in recent history, including a “Don’t Say Gay or Trans” law that was used to force a trans female teacher to go by “Mr.”
State Education Supt. Ryan Walters of Oklahoma was the latest to echo similar sentiments. Walters has recently appointed the right-wing media figure Chaya Raichik of Libs of TikTok to an advisory role “to improve school safety,” and notably, Raichik has posed proudly with papers accusing her of instigating bomb threats with her incendiary posts about LGBTQ people in classrooms.
The Title IX policies have been universally applauded by large LGBTQ rights organizations in the U.S. Lambda Legal, a key figure in fighting anti-LGBTQ legislation nationwide, said that the regulations “clearly cover LGBTQ+ students, as well as survivors and pregnant and parenting students across race and gender identity.” The Human Rights Campaign also praised the rule, stating, “rule will be life-changing for so many LGBTQ+ youth and help ensure LGBTQ+ students can receive the same educational experience as their peers: Going to dances, safely using the restroom, and writing stories that tell the truth about their own lives.”
The rule is slated to go into effect Aug. 1, pending any legal challenges.
****************************************************************************

Erin Reed is a transgender woman (she/her pronouns) and researcher who tracks anti-LGBTQ+ legislation around the world and helps people become better advocates for their queer family, friends, colleagues, and community. Reed also is a social media consultant and public speaker.
******************************************************************************************
The preceding article was first published at Erin In The Morning and is republished with permission.
The White House
White House debuts action plan targeting pollutants in drinking water
Same-sex couples face higher risk from environmental hazards

Headlining an Earth Day event in Northern Virginia’s Prince William Forest on Monday, President Joe Biden announced the disbursement of $7 billion in new grants for solar projects and warned of his Republican opponent’s plans to roll back the progress his administration has made toward addressing the harms of climate change.
The administration has led more than 500 programs geared toward communities most impacted by health and safety hazards like pollution and extreme weather events.
In a statement to the Washington Blade on Wednesday, Brenda Mallory, chair of the White House Council on Environmental Quality, said, “President Biden is leading the most ambitious climate, conservation, and environmental justice agenda in history — and that means working toward a future where all people can breathe clean air, drink clean water, and live in a healthy community.”
“This Earth Week, the Biden-Harris Administration announced $7 billion in solar energy projects for over 900,000 households in disadvantaged communities while creating hundreds of thousands of clean energy jobs, which are being made more accessible by the American Climate Corps,” she said. “President Biden is delivering on his promise to help protect all communities from the impacts of climate change — including the LGBTQI+ community — and that we leave no community behind as we build an equitable and inclusive clean energy economy for all.”
Recent milestones in the administration’s climate policies include the U.S. Environmental Protection Agency’s issuance on April 10 of legally enforceable standard for detecting and treating drinking water contaminated with polyfluoroalkyl substances.
“This rule sets health safeguards and will require public water systems to monitor and reduce the levels of PFAS in our nation’s drinking water, and notify the public of any exceedances of those levels,” according to a White House fact sheet. “The rule sets drinking water limits for five individual PFAS, including the most frequently found PFOA and PFOS.”
The move is expected to protect 100 million Americans from exposure to the “forever chemicals,” which have been linked to severe health problems including cancers, liver and heart damage, and developmental impacts in children.
An interactive dashboard from the United States Geological Survey shows the concentrations of polyfluoroalkyl substances in tapwater are highest in urban areas with dense populations, including cities like New York and Los Angeles.
During Biden’s tenure, the federal government has launched more than 500 programs that are geared toward investing in the communities most impacted by climate change, whether the harms may arise from chemical pollutants, extreme weather events, or other causes.
New research by the Williams Institute at the UCLA School of Law found that because LGBTQ Americans are likelier to live in coastal areas and densely populated cities, households with same-sex couples are likelier to experience the adverse effects of climate change.
The report notes that previous research, including a study that used “national Census data on same-sex households by census tract combined with data on hazardous air pollutants (HAPs) from the National Air Toxics Assessment” to model “the relationship between same-sex households and risk of cancer and respiratory illness” found “that higher prevalence of same-sex households is associated with higher risks for these diseases.”
“Climate change action plans at federal, state, and local levels, including disaster preparedness, response, and recovery plans, must be inclusive and address the specific needs and vulnerabilities facing LGBT people,” the Williams Institute wrote.
With respect to polyfluoroalkyl substances, the EPA’s adoption of new standards follows other federal actions undertaken during the Biden-Harris administration to protect firefighters and healthcare workers, test for and clean up pollution, and phase out or reduce use of the chemicals in fire suppressants, food packaging, and federal procurement.
The White House
Francisco Ruiz appointed director of White House Office of National AIDS Policy
Former CDC official is first Latino to run office

Francisco Ruiz’s appointment as the director of the White House Office of National AIDS Policy has elicited widespread acknowledgment across various sectors.
Ruiz, a distinguished figure in public health with a history of collaboration and strategic partnerships, assumes the role as the first-ever Latino to serve as ONAP’s director, underscoring a commitment to diversity and inclusivity in addressing public health challenges.
In response to his appointment, Domestic Policy Advisor Neera Tanden underscored the Biden-Harris administration’s steadfast commitment to ending the HIV epidemic and enhancing the quality of life for people living with HIV. Ruiz himself acknowledged this sentiment, emphasizing that accelerating efforts to combat the HIV epidemic and improve the well-being of those affected remain a paramount public health priority for the White House.
Previously serving at the U.S. Centers for Disease Control and Prevention, Ruiz played a pivotal role in advancing national HIV prevention campaigns, particularly contributing to the goals of the Ending the HIV Epidemic in the U.S. Initiative. His experience in fostering strategic partnerships and ensuring sensitive prevention messaging has been noted as instrumental in reaching diverse communities across the country and in U.S. territories.
Ruiz in his new role will be tasked with accelerating efforts to end the HIV epidemic and improve the quality of life for people living with HIV.
Guillermo Chacón, president of the Latino Commission on AIDS and founder of the Hispanic Health Network, expressed confidence in Ruiz’s ability to advance the national strategy to end the HIV epidemic.
“Mr. Ruiz is a respected public health leader and a fitting choice to ensure that the Biden-Harris administration meets the goal of ending the HIV epidemic in the United States and U.S. Territories,” said Chacón.
“Francisco Ruiz’s appointment signifies a renewed focus on addressing health disparities and promoting health equity, particularly for historically marginalized and underserved communities,” he added. “As a person living with HIV and the son of Mexican immigrants, Ruiz brings personal insight and professional expertise to his new role, ensuring that strategies to combat HIV/AIDS are scientifically grounded and connected with the experiences of those most affected.”
-

 District of Columbia4 days ago
District of Columbia4 days agoCatching up with the asexuals and aromantics of D.C.
-

 South America4 days ago
South America4 days agoArgentina government dismisses transgender public sector employees
-

 Maine5 days ago
Maine5 days agoMaine governor signs transgender, abortion sanctuary bill into law
-

 Mexico3 days ago
Mexico3 days agoMexican Senate approves bill to ban conversion therapy











