National
White House rejects gay judicial nominee
Supporters urged Schumer to fight for attorney accused of anti-Christian remarks

The White House has rejected the recommended nomination of a New York attorney who would have become the first openly gay man to sit on the federal bench, because of comments he reportedly made about the Pledge of Allegiance and Christmas that were deemed anti-Christian.
In February, U.S. Sen. Chuck Schumer (D-N.Y.) recommended the nomination of Daniel Alter to serve as a judge for the U.S. District Court for the Southern District of New York. Presidents traditionally follow the guidance of senators from the state where there’s a vacancy for judicial nominations.
But informed sources told the Washington Blade that the White House rejected Alter’s nomination because of remarks he reportedly made regarding a case challenging inclusion of the phrase “under God” in the Pledge of Allegiance. In addition, the White House reportedly objected to remarks that Alter made suggesting that merchants not wish shoppers “Merry Christmas” during the holidays.
In a 2005 article published by Cybercast News Service, Alter is quoted as saying that a general holiday greeting is more appropriate and inclusive for retailers as opposed to saying “Merry Christmas.”
“It seems both from a business … and a community perspective, that if merchandisers were going to do that … they would try to wish those in the community who may not share in celebrating Christmas a happy holiday as well,” Alter is quoted as saying.
“Our diversity has made us great and will continue to make us great and [‘Merry Christmas’] undermines both the holiday spirit as well as the message I think Americans should be sending to each other,” Alter reportedly continued.
The 2005 quotes were apparently reprinted in a 2008 CNS article that is stored in the archives on the organization’s website.
Additionally, in a 2004 article published in The New Republic, Alter is quoted as saying the U.S. Supreme Court case Elk Grove United School District v. Newdow “was a good case at the wrong time.” The case challenged use of the “under God” phrase in the Pledge of Allegiance in public schools.
The article reported Alter was “relieved” the Supreme Court decision “left open a window for future challenges.” The Anti-Defamation League had filed a friend-of-the-court brief in support of the Newdow case.
“When the right case does come along,” Alter reportedly said, “We’re there.”
Alter was previously an assistant U.S. attorney for the Southern District of New York and specialized in First Amendment and terrorism issues. He also served as national director of the civil rights division of the Anti-Defamation League, an organization that works to fight anti-Semitism.
The comments he reportedly made came in his capacity as an official with the Anti-Defamation League. The White House decision to reject Alter disappointed his supporters, who rallied around him and urged Schumer to advance his nomination anyway.
Schumer announced his recommended nomination of Alter during a Human Rights Campaign dinner in New York City and emphasized that his selection would make him the first openly gay male judge on the federal bench.
In a February statement, Schumer said he recommended Alter because he’s “a brilliant attorney who possesses the knowledge, balanced views and temperament required of a federal judge.”
“His outstanding leadership skills, his commitment to justice, and his extensive experience make him an exceptional choice for a position on the federal bench,” Schumer said. “I’m proud to nominate Daniel Alter. Period. But I am equally proud to nominate him because he is a history-maker who will be the first openly gay male judge in American history.”
But based on those reported statements, the White House and Schumer determined that Alter wouldn’t be able to reach the 60-vote threshold needed in the Senate to overcome a filibuster of his nomination. It’s unclear when the decision to reject Alter was made.
Schumer’s office didn’t respond to multiple requests for comment. A White House spokesperson declined to comment. Alter also declined to comment for this story.
Deborah Lauter, director of civil rights for the Anti-Defamation League, said the apparent decision to reject Alter’s nomination based on reported comments he made on behalf of the organization is “just plain unfair and unjust.”
“Any statements he made in the course of his job with ADL were just that — he was representing the views of our organization,” she said. “It’s dismaying if in fact that led to the derailing of his nomination.”
Lauter said Alter doesn’t recall speaking to The New Republic for the 2004 article and that Alter was misquoted in the 2005 CNS article.
“It was an inaccurate report and ADL should have insisted the record be corrected at the time,” Lauter said.
Lauter clarified that the Anti-Defamation League has never objected to retailers wishing customers “Merry Christmas.”
“But the bottom line is even if he made the comment, which he didn’t, it shouldn’t have disqualified him from service as a judge,” she said.
The decision to refuse the Alter nomination likely came sometime before July, when his supporters urged Schumer to go to bat for his recommended nominee.
In a letter dated July 2, 2010, a group of 66 attorneys who worked with Alter at the U.S. Attorney’s Office for the Southern District of New York wrote that the designation of Alter to the federal bench is “a nomination worth fighting for.”
“We urge you to take all possible steps to ensure that Mr. Alter is nominated to the federal bench and promptly considered by the Senate Judiciary Committee,” the letter states.
Among those who signed the letter is James Comey, who served as deputy attorney general during the Republican administration of former President George W. Bush.
The attorneys wrote that Alter’s “nomination to the federal bench is in jeopardy” because of “demonstrably false statements” that reporters made while he was working for the Anti-Defamation League. The missive doesn’t detail why the statements Alter reportedly made to media outlets are “demonstrably false.”
“While we will let others set forth the factual reasons why these allegations are baseless, we write to state emphatically that the sentiments falsely ascribed to Mr. Alter are inconsistent with everything that we know about him,” the letter states. “Mr. Alter has dedicated his life to tolerance, public service, moderation, and fidelity to law. He is unfailingly kind, respectful, and open-minded. In both deed and character, Mr. Alter is the antithesis of the views that have been misattributed to him.”
The signers state that they “cannot imagine a more highly qualified nominee” and that the loss of Alter to the federal judiciary based on “false allegations” would be significant.
“By temperament, he is well-suited to the bench, possessing every quality one seeks in a judge: respect for all views, dedication to the public, tireless pursuit of the best legal argument, and a determination to reach decisions that will command the respect of all parties,” the letter states.
Lauter said the Anti-Defamation League sent its own letter to Schumer in July urging the senator to push for Alter’s nomination, but she declined to make the letter public.
“It was a private letter to the senator just clarifying the record and expressing support — enthusiastically and without reservation — for Danny Alter’s nomination,” she said.
Also lamenting the derailment of Alter’s nomination is Richard Socarides, a gay New York attorney who served as an adviser to President Clinton.
Socarides told the Blade the White House’s rejection of Alter’s nomination was evidence of a broken system.
“I don’t know Daniel Alter personally,” Socarides said. “I’m told he is highly qualified. We need more people like him in the federal judiciary. I don’t know why his nomination got derailed, but certainly a system in which someone like Alter can’t get confirmed is badly broken.”
HRC heralded Schumer’s announcement of his recommended nomination of Alter in February, but the organization is mum on his rejection.
At the time of the announcement, Joe Solmonese, HRC’s president, said in a statement that Alter “is eminently qualified for a position on the federal bench.”
“America is taking a step forward toward equality by evaluating an individual based on his accomplishments and without regard to his sexual orientation,” Solmonese said. “We commend Senator Schumer for his historic recommendation, and look forward to the President’s nomination.”
Fred Sainz, HRC’s vice president of communications, this week declined to comment on the White House rejection of Alter.
Schumer has since recommended the nomination of another openly gay man, J. Paul Oetken, to become a district judge for the U.S. District Court for the Southern District of New York.
The New York senator made the announcement in a Sept. 23 statement that said Oetken has “the right combination of skills, experience and dedication to [be] an excellent judge on the court.”
Oetken served as an attorney in private practice and was an associate counsel for former President Bill Clinton, according to the Schumer statement.
Pennsylvania
Malcolm Kenyatta could become the first LGBTQ statewide elected official in Pa.
State lawmaker a prominent Biden-Harris 2024 reelection campaign surrogate

Following his win in the Democratic primary contest on Wednesday, Pennsylvania state Rep. Malcolm Kenyatta, who is running for auditor general, is positioned to potentially become the first openly LGBTQ elected official serving the commonwealth.
In a statement celebrating his victory, LGBTQ+ Victory Fund President Annise Parker said, “Pennsylvanians trust Malcolm Kenyatta to be their watchdog as auditor general because that’s exactly what he’s been as a legislator.”
“LGBTQ+ Victory Fund is all in for Malcolm, because we know he has the experience to win this race and carry on his fight for students, seniors and workers as Pennsylvania’s auditor general,” she said.
Parker added, “LGBTQ+ Americans are severely underrepresented in public office and the numbers are even worse for Black LGBTQ+ representation. I look forward to doing everything I can to mobilize LGBTQ+ Pennsylvanians and our allies to get out and vote for Malcolm this November so we can make history.”
In April 2023, Kenyatta was appointed by the White House to serve as director of the Presidential Advisory Commission on Advancing Educational Equity, Excellence and Economic Opportunity for Black Americans.
He has been an active surrogate in the Biden-Harris 2024 reelection campaign.
The White House
White House debuts action plan targeting pollutants in drinking water
Same-sex couples face higher risk from environmental hazards

Headlining an Earth Day event in Northern Virginia’s Prince William Forest on Monday, President Joe Biden announced the disbursement of $7 billion in new grants for solar projects and warned of his Republican opponent’s plans to roll back the progress his administration has made toward addressing the harms of climate change.
The administration has led more than 500 programs geared toward communities most impacted by health and safety hazards like pollution and extreme weather events.
In a statement to the Washington Blade on Wednesday, Brenda Mallory, chair of the White House Council on Environmental Quality, said, “President Biden is leading the most ambitious climate, conservation, and environmental justice agenda in history — and that means working toward a future where all people can breathe clean air, drink clean water, and live in a healthy community.”
“This Earth Week, the Biden-Harris Administration announced $7 billion in solar energy projects for over 900,000 households in disadvantaged communities while creating hundreds of thousands of clean energy jobs, which are being made more accessible by the American Climate Corps,” she said. “President Biden is delivering on his promise to help protect all communities from the impacts of climate change — including the LGBTQI+ community — and that we leave no community behind as we build an equitable and inclusive clean energy economy for all.”
Recent milestones in the administration’s climate policies include the U.S. Environmental Protection Agency’s issuance on April 10 of legally enforceable standard for detecting and treating drinking water contaminated with polyfluoroalkyl substances.
“This rule sets health safeguards and will require public water systems to monitor and reduce the levels of PFAS in our nation’s drinking water, and notify the public of any exceedances of those levels,” according to a White House fact sheet. “The rule sets drinking water limits for five individual PFAS, including the most frequently found PFOA and PFOS.”
The move is expected to protect 100 million Americans from exposure to the “forever chemicals,” which have been linked to severe health problems including cancers, liver and heart damage, and developmental impacts in children.
An interactive dashboard from the United States Geological Survey shows the concentrations of polyfluoroalkyl substances in tapwater are highest in urban areas with dense populations, including cities like New York and Los Angeles.
During Biden’s tenure, the federal government has launched more than 500 programs that are geared toward investing in the communities most impacted by climate change, whether the harms may arise from chemical pollutants, extreme weather events, or other causes.
New research by the Williams Institute at the UCLA School of Law found that because LGBTQ Americans are likelier to live in coastal areas and densely populated cities, households with same-sex couples are likelier to experience the adverse effects of climate change.
The report notes that previous research, including a study that used “national Census data on same-sex households by census tract combined with data on hazardous air pollutants (HAPs) from the National Air Toxics Assessment” to model “the relationship between same-sex households and risk of cancer and respiratory illness” found “that higher prevalence of same-sex households is associated with higher risks for these diseases.”
“Climate change action plans at federal, state, and local levels, including disaster preparedness, response, and recovery plans, must be inclusive and address the specific needs and vulnerabilities facing LGBT people,” the Williams Institute wrote.
With respect to polyfluoroalkyl substances, the EPA’s adoption of new standards follows other federal actions undertaken during the Biden-Harris administration to protect firefighters and healthcare workers, test for and clean up pollution, and phase out or reduce use of the chemicals in fire suppressants, food packaging, and federal procurement.
Maine
Maine governor signs transgender, abortion sanctuary bill into law
Bomb threats made against lawmakers before measure’s passage
BY ERIN REED | On Tuesday, Maine Gov. Janet Mills signed LD 227, a sanctuary bill that protects transgender and abortion providers and patients from out-of-state prosecution, into law.
With this action, Maine becomes the 16th state to explicitly protect trans and abortion care in state law from prosecution. This follows several bomb threats targeting state legislators after social media attacks from far-right anti-trans influencers such as Riley Gaines and Chaya Raichik of Libs of TikTok.
An earlier version of the bill failed in committee after similar attacks in January. Undeterred, Democrats reconvened and added additional protections to the bill before it was passed into law.
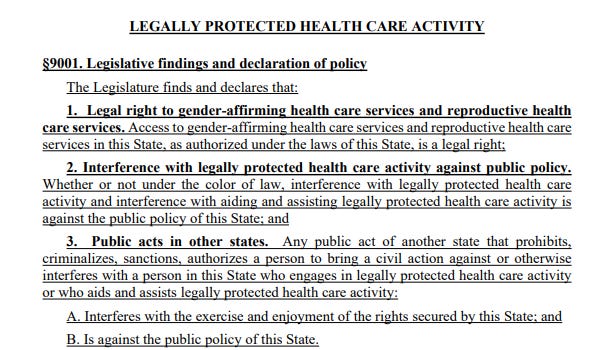
The law is extensive. It asserts that gender-affirming care and reproductive health care are “legal rights” in Maine. It states that criminal and civil actions against providers and patients are not enforceable if the provision or access to that care occurred within Maine’s borders, asserting jurisdiction over those matters.
It bars cooperation with out-of-state subpoenas and arrest warrants for gender-affirming care and abortion that happen within the state. It even protects doctors who provide gender-affirming care and abortion from certain adverse actions by medical boards, malpractice insurance, and other regulating entities, shielding those providers from attempts to economically harm them through out-of-state legislation designed to dissuade them from providing care.
You can see the findings section of the bill here:
The bill also explicitly enshrines the World Professional Association of Transgender Health’s Standards of Care, which have been the target of right-wing disinformation campaigns, into state law for the coverage of trans healthcare:
The bill is said to be necessary due to attempts to prosecute doctors and seek information from patients across state lines. In recent months, attorneys general in other states have attempted to obtain health care data on trans patients who traveled to obtain care. According to the U.S. Senate Finance Committee, attorneys general in Tennessee, Indiana, Missouri, and Texas attempted to obtain detailed medical records “to terrorize transgender teens in their states … opening the door to criminalizing women’s private reproductive health care choices.”
The most blatant of these attempts was from the attorney general of Texas, who, according to the Senate Finance Committee, “sent demands to at least two non-Texas entities.” One of these entities was Seattle Children’s Hospital, which received a letter threatening administrators with arrest unless they sent data on Texas patients traveling to Seattle to obtain gender-affirming care.
Seattle Children’s Hospital settled that case out of court this week, agreeing to withdraw its Texas business registration in return for Texas dropping its investigation. This likely will have no impact on Seattle Children’s Hospital, which has stated it did not treat any youth via telemedicine or in person in Texas; the hospital will be able to continue treating Texas youth who travel outside of Texas to obtain their care. That settlement was likely compelling due to a nearly identical law in Washington that barred out-of-state investigations on trans care obtained solely in the state of Washington.
The bill has faced a rocky road to passage. A similar bill was debated in January, but after coming under intense attack from anti-trans activists who misleadingly called it a “transgender trafficking bill,” the bill was voluntarily withdrawn by its sponsor.
When LD 227 was introduced, it faced even more attacks from Gaines and Libs of TikTok. These attacks were followed by bomb threats that forced the evacuation of the legislature, promising “death to pedophiles” and stating that a bomb would detonate within a few hours in the capitol building.
Despite these threats, legislators strengthened both the abortion and gender-affirming care provisions and pressed forward, passing the bill into law. Provisions found in the new bill include protecting people who “aid and assist” gender-affirming care and abortion, protections against court orders from other states for care obtained in Maine, and even protections against adverse actions by health insurance and malpractice insurance providers, which have been recent targets of out-of-state legislation aimed at financially discouraging doctors from providing gender-affirming care and abortion care even in states where it is legal.
See a few of the extensive health insurance and malpractice provisions here:
Speaking about the bill, Gia Drew, executive director of Equality Maine, said in a statement, “We are thrilled to see LD 227, the shield bill, be signed into law by Gov. Mills. Thanks to our pro equality and pro reproductive choice elected officials who refused to back down in the face of disinformation. This bill couldn’t come into effect at a better time, as more than 40 percent of states across the country have either banned or attempted to block access to reproductive care, which includes abortions, as well as transgender healthcare for minors. Thanks to our coalition partners who worked tirelessly to phone bank, lobby, and get this bill over the finish line to protect community health.”
Destie Hohman Sprague of the Maine Women’s Lobby celebrated the passage of the bill despite threats of violence, saying in a statement, “A gender-just Maine ensures that all Mainers have access to quality health care that supports their mental and physical wellbeing and bodily autonomy, including comprehensive reproductive and gender-affirming care. We celebrate the passage of LD 227, which helps us meet that goal. Still, the patterns of violence and disinformation ahead of the vote reflected the growing connections between misogyny, extremism, and anti-democratic threats and actions. We must continue to advocate for policies that protect bodily autonomy, and push back against extremist rhetoric that threatens our states’ rights and our citizens’ freedoms.”
The decision to pass the legislation comes as the Biden administration released updated HIPAA protections that protect “reproductive health care” from out-of-state prosecutions and investigations.
Although the definition of “reproductive health care” is broad in the new HIPAA regulations, it is uncertain whether they will include gender-affirming care. For at least 16 states, though, gender-affirming care is now explicitly protected by state law and shielded from out-of-state legislation, providing trans people and those seeking abortions with protections as the fight increasingly crosses state lines.
****************************************************************************

Erin Reed is a transgender woman (she/her pronouns) and researcher who tracks anti-LGBTQ+ legislation around the world and helps people become better advocates for their queer family, friends, colleagues, and community. Reed also is a social media consultant and public speaker.
******************************************************************************************
The preceding article was first published at Erin In The Morning and is republished with permission.
-

 State Department2 days ago
State Department2 days agoState Department releases annual human rights report
-

 Maryland4 days ago
Maryland4 days agoJoe Vogel campaign holds ‘Big Gay Canvass Kickoff’
-

 Politics3 days ago
Politics3 days agoSmithsonian staff concerned about future of LGBTQ programming amid GOP scrutiny
-

 The White House1 day ago
The White House1 day agoWhite House debuts action plan targeting pollutants in drinking water