National
HISTORIC: Federal appeals court strikes down DOMA
Judges unanimously rule 3-0 against anti-gay law
A significant blow came to the Defense of Marriage Act on Thursday when for the first time a federal appeals court ruled the anti-gay law was unconstitutional.
In a unanimous 3-0 decision, the U.S. First Circuit Court of Appeals in Massachusetts ruled that Section 3 of DOMA, which prohibit federal recognition of same-sex marriage, violated married same-sex couples rights under the U.S. Constitution.
“To conclude, many Americans believe that marriage is the union of a man and a woman, and most Americans live in states where that is the law today,” the decision states. “One virtue of federalism is that it permits this diversity of governance based on local choice, but this applies as well to the states that have chosen to legalize same-sex marriage. Under current Supreme Court authority, Congress’ denial of federal benefits to same-sex couples lawfully married in Massachusetts has not been adequately supported by any permissible federal interest.”
The ruling was written U.S. Circuit Court Judge Michael Boudin, who heard the case along with Chief Circuit Judge Sandra Lynch and Circuit Judge Juan Torruella.
Lynch was appointed by a Democrat, former President Bill Clinton, but the other judges were appointed by Republicans. Torruella was appointed by former President Ronald Reagan and Boudin was appointed by former President George H.W. Bush.
The decision was brought down in two cases challenging DOMA: Gill v. Office of Personnel Management, which was filed by Gay & Lesbian Advocates & Defenders; and Commonwealth of Massachusetts v. Department of Health & Human Services, which was filed by Massachusetts Attorney General Martha Coakley.
The Gill lawsuit contends that DOMA is unconstitutional because it violates equal protection under the U.S. Constitution, while Commonwealth lawsuit argues DOMA violates a state’s right to regulate marriage under the Tenth Amendment.
In a statement, Coakley praised the ruling as evidence that DOMA violates married gay couples’ rights under the U.S. Constitution.
“Today’s landmark ruling makes clear once again that DOMA is a discriminatory law for which there is no justification,” Coakley said. “It is unconstitutional for the federal government to create a system of first- and second-class marriages, and it does harm to families in Massachusetts every day. All Massachusetts couples should be afforded the same rights and protections under the law, and we hope that this decision will be the final step toward ensuring that equality for all.”
Retiring gay Rep. Barney Frank, a Democrat who represents Massachusetts in Congress, also praised the ruling and expressed confidence the ruling against DOMA will be upheld when the case reaches the Supreme Court.
“The current situation, in which the rights of some couples married in other states are recognized, while the rights of other couples married in those states are denied, is clearly a violation of the U.S. Constitution,” Frank said. “I am confident that the U.S. Supreme Court will add its support for this decision which is so firmly grounded in long-standing American constitutional principles.”
The appeals court ruling affirms U.S. District Court Judge Joseph Tauro’s decision against DOMA in the two decisions he delivered in response to the lawsuits in July 2010. Since that time, President Obama has discontinued defending DOMA in court. Two other federal courts have also concluded that DOMA is unconstitutional.
Douglas NeJaime, who’s gay and a law professor at Loyola Law School, called the First Circuit decision “concise and relatively straightforward” and said the bigger question is whether this case or the challenge against California’s Proposition 8, Perry v. Brown, will first reach the Supreme Court.
“Whichever case makes it there first will have significant consequences for the same-sex marriage movement,” NeJaime said. “If Gill gives the Court its first bite at the same-sex marriage apple, the justices will address the more limited and potentially less controversial question of the federal rights of same-sex couples who are already married. Whereas Perry could present the Justices with broader questions of the rights of same-sex couples to marry, potentially across the nation.”
The Bipartisan Legal Advisory Group, under the direction of House Speaker John Boehner, voted on a party-line basis to take up defense of DOMA in the administration’s stead. It’s likely to appeal the decision either to the full First Circuit or the U.S. Supreme Court.
Oral arguments before the First Circuit took place April 4. Mary Bonauto, GLAD’s civil rights project director, represented her organization during the hearing. Massachusetts Assistant Attorney General Maura Healey argued on behalf of Massachusetts.
Defending DOMA in court was Paul Clement, a former U.S. solicitor general. After the Obama administration declared it would no longer defend DOMA, House Speaker John Boehner hired Clement to advocate for DOMA on behalf of the Bipartisan Legal Advisory Group, which voted along party lines to take up defense of the law.
Stuart Delery, who’s gay and the Justice Department’s acting assistant attorney general for the civil division, also litigated against the anti-gay law in court.
NOTE: This post has been edited and updated.
Pennsylvania
Malcolm Kenyatta could become the first LGBTQ statewide elected official in Pa.
State lawmaker a prominent Biden-Harris 2024 reelection campaign surrogate

Following his win in the Democratic primary contest on Wednesday, Pennsylvania state Rep. Malcolm Kenyatta, who is running for auditor general, is positioned to potentially become the first openly LGBTQ elected official serving the commonwealth.
In a statement celebrating his victory, LGBTQ+ Victory Fund President Annise Parker said, “Pennsylvanians trust Malcolm Kenyatta to be their watchdog as auditor general because that’s exactly what he’s been as a legislator.”
“LGBTQ+ Victory Fund is all in for Malcolm, because we know he has the experience to win this race and carry on his fight for students, seniors and workers as Pennsylvania’s auditor general,” she said.
Parker added, “LGBTQ+ Americans are severely underrepresented in public office and the numbers are even worse for Black LGBTQ+ representation. I look forward to doing everything I can to mobilize LGBTQ+ Pennsylvanians and our allies to get out and vote for Malcolm this November so we can make history.”
In April 2023, Kenyatta was appointed by the White House to serve as director of the Presidential Advisory Commission on Advancing Educational Equity, Excellence and Economic Opportunity for Black Americans.
He has been an active surrogate in the Biden-Harris 2024 reelection campaign.
The White House
White House debuts action plan targeting pollutants in drinking water
Same-sex couples face higher risk from environmental hazards

Headlining an Earth Day event in Northern Virginia’s Prince William Forest on Monday, President Joe Biden announced the disbursement of $7 billion in new grants for solar projects and warned of his Republican opponent’s plans to roll back the progress his administration has made toward addressing the harms of climate change.
The administration has led more than 500 programs geared toward communities most impacted by health and safety hazards like pollution and extreme weather events.
In a statement to the Washington Blade on Wednesday, Brenda Mallory, chair of the White House Council on Environmental Quality, said, “President Biden is leading the most ambitious climate, conservation, and environmental justice agenda in history — and that means working toward a future where all people can breathe clean air, drink clean water, and live in a healthy community.”
“This Earth Week, the Biden-Harris Administration announced $7 billion in solar energy projects for over 900,000 households in disadvantaged communities while creating hundreds of thousands of clean energy jobs, which are being made more accessible by the American Climate Corps,” she said. “President Biden is delivering on his promise to help protect all communities from the impacts of climate change — including the LGBTQI+ community — and that we leave no community behind as we build an equitable and inclusive clean energy economy for all.”
Recent milestones in the administration’s climate policies include the U.S. Environmental Protection Agency’s issuance on April 10 of legally enforceable standard for detecting and treating drinking water contaminated with polyfluoroalkyl substances.
“This rule sets health safeguards and will require public water systems to monitor and reduce the levels of PFAS in our nation’s drinking water, and notify the public of any exceedances of those levels,” according to a White House fact sheet. “The rule sets drinking water limits for five individual PFAS, including the most frequently found PFOA and PFOS.”
The move is expected to protect 100 million Americans from exposure to the “forever chemicals,” which have been linked to severe health problems including cancers, liver and heart damage, and developmental impacts in children.
An interactive dashboard from the United States Geological Survey shows the concentrations of polyfluoroalkyl substances in tapwater are highest in urban areas with dense populations, including cities like New York and Los Angeles.
During Biden’s tenure, the federal government has launched more than 500 programs that are geared toward investing in the communities most impacted by climate change, whether the harms may arise from chemical pollutants, extreme weather events, or other causes.
New research by the Williams Institute at the UCLA School of Law found that because LGBTQ Americans are likelier to live in coastal areas and densely populated cities, households with same-sex couples are likelier to experience the adverse effects of climate change.
The report notes that previous research, including a study that used “national Census data on same-sex households by census tract combined with data on hazardous air pollutants (HAPs) from the National Air Toxics Assessment” to model “the relationship between same-sex households and risk of cancer and respiratory illness” found “that higher prevalence of same-sex households is associated with higher risks for these diseases.”
“Climate change action plans at federal, state, and local levels, including disaster preparedness, response, and recovery plans, must be inclusive and address the specific needs and vulnerabilities facing LGBT people,” the Williams Institute wrote.
With respect to polyfluoroalkyl substances, the EPA’s adoption of new standards follows other federal actions undertaken during the Biden-Harris administration to protect firefighters and healthcare workers, test for and clean up pollution, and phase out or reduce use of the chemicals in fire suppressants, food packaging, and federal procurement.
Maine
Maine governor signs transgender, abortion sanctuary bill into law
Bomb threats made against lawmakers before measure’s passage
BY ERIN REED | On Tuesday, Maine Gov. Janet Mills signed LD 227, a sanctuary bill that protects transgender and abortion providers and patients from out-of-state prosecution, into law.
With this action, Maine becomes the 16th state to explicitly protect trans and abortion care in state law from prosecution. This follows several bomb threats targeting state legislators after social media attacks from far-right anti-trans influencers such as Riley Gaines and Chaya Raichik of Libs of TikTok.
An earlier version of the bill failed in committee after similar attacks in January. Undeterred, Democrats reconvened and added additional protections to the bill before it was passed into law.
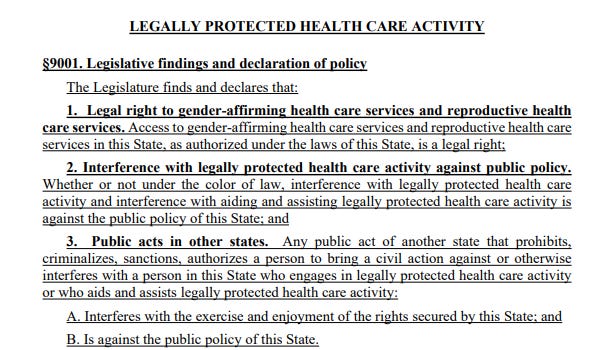
The law is extensive. It asserts that gender-affirming care and reproductive health care are “legal rights” in Maine. It states that criminal and civil actions against providers and patients are not enforceable if the provision or access to that care occurred within Maine’s borders, asserting jurisdiction over those matters.
It bars cooperation with out-of-state subpoenas and arrest warrants for gender-affirming care and abortion that happen within the state. It even protects doctors who provide gender-affirming care and abortion from certain adverse actions by medical boards, malpractice insurance, and other regulating entities, shielding those providers from attempts to economically harm them through out-of-state legislation designed to dissuade them from providing care.
You can see the findings section of the bill here:
The bill also explicitly enshrines the World Professional Association of Transgender Health’s Standards of Care, which have been the target of right-wing disinformation campaigns, into state law for the coverage of trans healthcare:
The bill is said to be necessary due to attempts to prosecute doctors and seek information from patients across state lines. In recent months, attorneys general in other states have attempted to obtain health care data on trans patients who traveled to obtain care. According to the U.S. Senate Finance Committee, attorneys general in Tennessee, Indiana, Missouri, and Texas attempted to obtain detailed medical records “to terrorize transgender teens in their states … opening the door to criminalizing women’s private reproductive health care choices.”
The most blatant of these attempts was from the attorney general of Texas, who, according to the Senate Finance Committee, “sent demands to at least two non-Texas entities.” One of these entities was Seattle Children’s Hospital, which received a letter threatening administrators with arrest unless they sent data on Texas patients traveling to Seattle to obtain gender-affirming care.
Seattle Children’s Hospital settled that case out of court this week, agreeing to withdraw its Texas business registration in return for Texas dropping its investigation. This likely will have no impact on Seattle Children’s Hospital, which has stated it did not treat any youth via telemedicine or in person in Texas; the hospital will be able to continue treating Texas youth who travel outside of Texas to obtain their care. That settlement was likely compelling due to a nearly identical law in Washington that barred out-of-state investigations on trans care obtained solely in the state of Washington.
The bill has faced a rocky road to passage. A similar bill was debated in January, but after coming under intense attack from anti-trans activists who misleadingly called it a “transgender trafficking bill,” the bill was voluntarily withdrawn by its sponsor.
When LD 227 was introduced, it faced even more attacks from Gaines and Libs of TikTok. These attacks were followed by bomb threats that forced the evacuation of the legislature, promising “death to pedophiles” and stating that a bomb would detonate within a few hours in the capitol building.
Despite these threats, legislators strengthened both the abortion and gender-affirming care provisions and pressed forward, passing the bill into law. Provisions found in the new bill include protecting people who “aid and assist” gender-affirming care and abortion, protections against court orders from other states for care obtained in Maine, and even protections against adverse actions by health insurance and malpractice insurance providers, which have been recent targets of out-of-state legislation aimed at financially discouraging doctors from providing gender-affirming care and abortion care even in states where it is legal.
See a few of the extensive health insurance and malpractice provisions here:
Speaking about the bill, Gia Drew, executive director of Equality Maine, said in a statement, “We are thrilled to see LD 227, the shield bill, be signed into law by Gov. Mills. Thanks to our pro equality and pro reproductive choice elected officials who refused to back down in the face of disinformation. This bill couldn’t come into effect at a better time, as more than 40 percent of states across the country have either banned or attempted to block access to reproductive care, which includes abortions, as well as transgender healthcare for minors. Thanks to our coalition partners who worked tirelessly to phone bank, lobby, and get this bill over the finish line to protect community health.”
Destie Hohman Sprague of the Maine Women’s Lobby celebrated the passage of the bill despite threats of violence, saying in a statement, “A gender-just Maine ensures that all Mainers have access to quality health care that supports their mental and physical wellbeing and bodily autonomy, including comprehensive reproductive and gender-affirming care. We celebrate the passage of LD 227, which helps us meet that goal. Still, the patterns of violence and disinformation ahead of the vote reflected the growing connections between misogyny, extremism, and anti-democratic threats and actions. We must continue to advocate for policies that protect bodily autonomy, and push back against extremist rhetoric that threatens our states’ rights and our citizens’ freedoms.”
The decision to pass the legislation comes as the Biden administration released updated HIPAA protections that protect “reproductive health care” from out-of-state prosecutions and investigations.
Although the definition of “reproductive health care” is broad in the new HIPAA regulations, it is uncertain whether they will include gender-affirming care. For at least 16 states, though, gender-affirming care is now explicitly protected by state law and shielded from out-of-state legislation, providing trans people and those seeking abortions with protections as the fight increasingly crosses state lines.
****************************************************************************

Erin Reed is a transgender woman (she/her pronouns) and researcher who tracks anti-LGBTQ+ legislation around the world and helps people become better advocates for their queer family, friends, colleagues, and community. Reed also is a social media consultant and public speaker.
******************************************************************************************
The preceding article was first published at Erin In The Morning and is republished with permission.