homepage news
FDA notice suggests reconsideration of gay blood ban
Soliciting public feedback on deferral policy

The Food & Drug Administration has announced it is seeking comment on deferral policies for blood donations, suggesting it’s reconsidering the prohibition of blood donations from gay and bisexual men.
In a seven-page notice set for official publication in the Federal Register on Thursday, the agency indicates it’s opening a public comment period on policies intended to keep HIV out of the blood supply.
“As part of the effort to continue to assess its donor deferral policies, FDA is opening this docket to provide a mechanism for the public to submit additional information regarding potential blood donor deferral policy options,” the notice says. “Specifically, we invite interested persons to submit to the docket comments supported by scientific evidence regarding possible revisions to FDA’s blood donor deferral policies to reduce the risk of HIV transmission by blood and blood products.”
Currently, the Food & Drug Administration requires gay and bisexual men to abstain from sex with other men for one year before donating blood. That policy, implemented last year, replaced the lifetime ban on blood donations from men who have had sex with men, although LGBT advocates have said the new deferral requirement is still essentially a ban.
Observing that opponents of the current policy of deferral have sought to replace it with one that evaluates individual risk, the FDA poses six questions to potential commenters and encourages stakeholders to provide scientific date to back up their comments:
1. What questions would most effectively identify individuals at risk of transmitting HIV through blood donation?
2. Are there specific questions that could be asked that might best capture the recent risk of a donor acquiring HIV infection, such as within the 2 to 4 weeks immediately preceding blood donation?
3. How specific can the questions be regarding sexual practices while remaining understandable and acceptable to all blood donors? For example, could questions about specific sexual behaviors be asked if they helped to identify which donors should be at least temporarily deferred because of risk factors? To the extent the questions are explicit about sexual practices, how willing will donors be to answer such questions accurately?
4. Under what circumstances would a short deferral period for high risk behavior be appropriate? For each short deferral period identified, please specify the duration of the deferral and provide the scientific rationale.
5. What changes might be necessary within blood collection establishments to assure that accurate, individual HIV risk assessments are performed?
6. How best to design a potential study to evaluate the feasibility and effectiveness of alternative deferral options such as individual risk assessment?
Tara Goodin, an FDA spokesperson, said the notice establishes a “formal process” by which the agency solicits comment on the idea of moving from current policy “to alternate deferral options including the use of individual risk assessment.”
“The recommendations revised in December 2015, which are based on risk behaviors and not sexual orientation, are founded upon the best scientific information currently available, and included a rigorous examination of several alternative options, including individual risk assessment,” Goodin said. “Opening of the public document means that we’re seeking information and data on the feasibility of potentially moving toward individual risk assessments, if scientific evidence supports such a change.”
U.S. Sen. Tammy Baldwin (D-Wis.), the only out lesbian in Congress, said in a statement the notice is an “encouraging” step in an effort to lift the gay and bisexual blood ban.
“I have long fought to end discriminatory blood donation policies and improve them, including for healthy gay and bisexual men,” Baldwin said. “It is encouraging that the FDA is taking another step forward to develop better blood donor policies that are grounded in science, don’t unfairly single out one group of individuals, and allow all healthy Americans to donate. I will continue to push for policies that secure our nation’s blood supply in a scientifically sound manner based on individual risk.”
In the aftermath of the mass shooting at a gay nightclub in Orlando, Fla., last month, which claimed the lives of 49 people and wounded 53 others, lawmakers had called on the Obama administration to lift the ban in favor of a policy based on individual risk.
In June, Baldwin was among 24 U.S. senators to sign a letter urging the Department of Health & Human Services to change the policy. In a separate letter, U.S. Reps. Barbara Lee (D-Calif.), Mike Quigley (D-Ill.) and Debbie Wasserman Schultz (D-Fla.) were among more than 100 House lawmakers who signed a separate letter calling for an end to gay and bisexual blood ban.
White House Press Secretary Josh Earnest defended the one-year deferral requirement on blood donations from gay and bisexual men in the aftermath of the Orlando shooting as a policy based on “scientific advice.” On Tuesday, the White House didn’t respond to the Washington Blade’s request to comment on the notice.
Brandon Lorenz, a spokesperson for the Human Rights Campaign, said his organization is hopeful the FDA’s request for comment indicates an upcoming change in policy.
“The FDA’s current policy cannot be justified in light of current scientific research and updated blood screening technology,” Lorenz said. “We are committed to working towards an outcome that both minimizes risk to the blood supply and treats gay and bisexual men with the respect they deserve. We’re hopeful this call for comments represents the next step toward changing an outdated and short-sided policy.”
homepage news
Honoring the legacy of New Orleans’ 1973 UpStairs Lounge fire
Why the arson attack that killed 32 gay men still resonates 50 years later

On June 23 of last year, I held the microphone as a gay man in the New Orleans City Council Chamber and related a lost piece of queer history to the seven council members. I told this story to disabuse all New Orleanians of the notion that silence and accommodation, in the face of institutional and official failures, are a path to healing.
The story I related to them began on a typical Sunday night at a second-story bar on the fringe of New Orleans’ French Quarter in 1973, where working-class men would gather around a white baby grand piano and belt out the lyrics to a song that was the anthem of their hidden community, “United We Stand” by the Brotherhood of Man.
“United we stand,” the men would sing together, “divided we fall” — the words epitomizing the ethos of their beloved UpStairs Lounge bar, an egalitarian free space that served as a forerunner to today’s queer safe havens.
Around that piano in the 1970s Deep South, gays and lesbians, white and Black queens, Christians and non-Christians, and even early gender minorities could cast aside the racism, sexism, and homophobia of the times to find acceptance and companionship for a moment.
For regulars, the UpStairs Lounge was a miracle, a small pocket of acceptance in a broader world where their very identities were illegal.
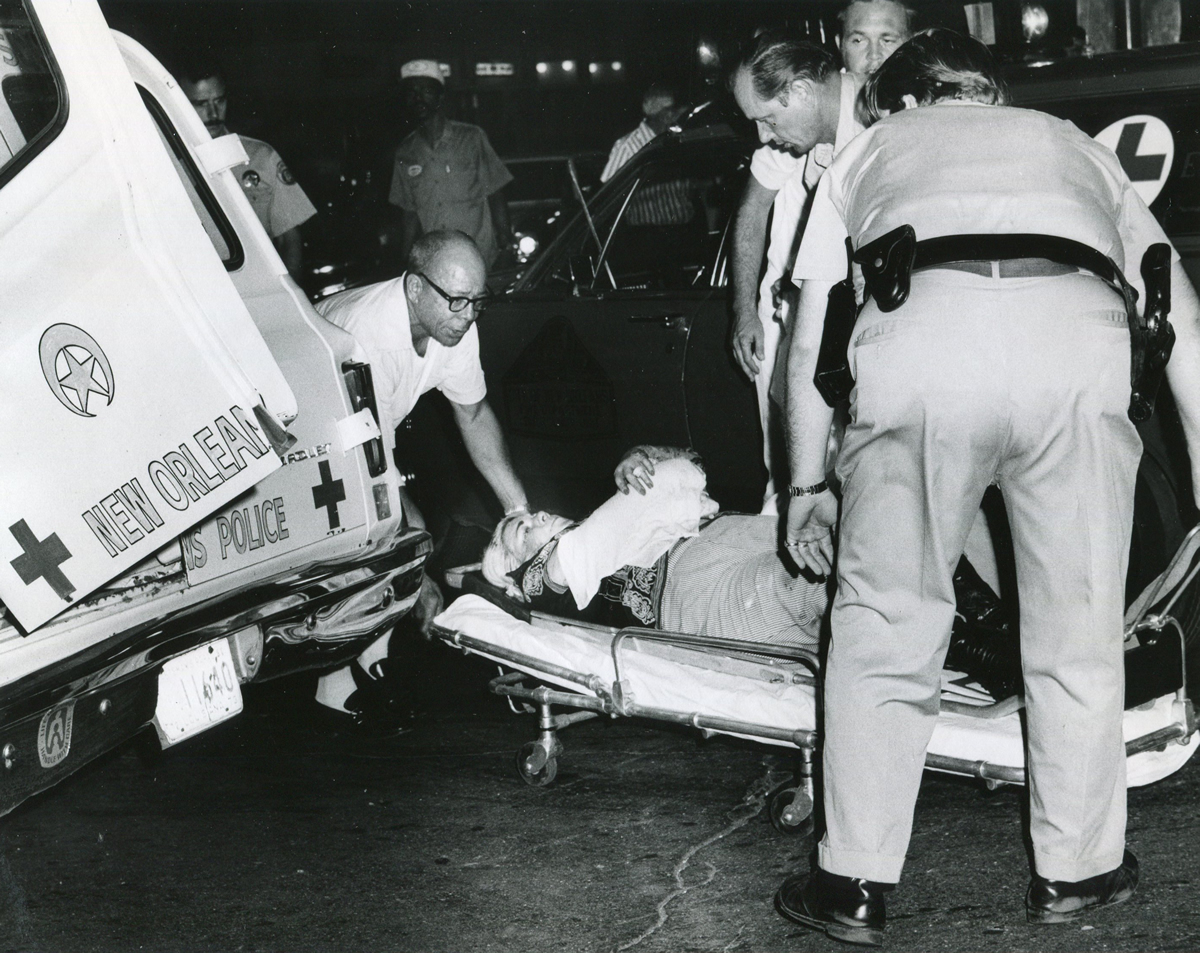
On the Sunday night of June 24, 1973, their voices were silenced in a murderous act of arson that claimed 32 lives and still stands as the deadliest fire in New Orleans history — and the worst mass killing of gays in 20th century America.
As 13 fire companies struggled to douse the inferno, police refused to question the chief suspect, even though gay witnesses identified and brought the soot-covered man to officers idly standing by. This suspect, an internally conflicted gay-for-pay sex worker named Rodger Dale Nunez, had been ejected from the UpStairs Lounge screaming the word “burn” minutes before, but New Orleans police rebuffed the testimony of fire survivors on the street and allowed Nunez to disappear.
As the fire raged, police denigrated the deceased to reporters on the street: “Some thieves hung out there, and you know this was a queer bar.”
For days afterward, the carnage met with official silence. With no local gay political leaders willing to step forward, national Gay Liberation-era figures like Rev. Troy Perry of the Metropolitan Community Church flew in to “help our bereaved brothers and sisters” — and shatter officialdom’s code of silence.
Perry broke local taboos by holding a press conference as an openly gay man. “It’s high time that you people, in New Orleans, Louisiana, got the message and joined the rest of the Union,” Perry said.
Two days later, on June 26, 1973, as families hesitated to step forward to identify their kin in the morgue, UpStairs Lounge owner Phil Esteve stood in his badly charred bar, the air still foul with death. He rebuffed attempts by Perry to turn the fire into a call for visibility and progress for homosexuals.
“This fire had very little to do with the gay movement or with anything gay,” Esteve told a reporter from The Philadelphia Inquirer. “I do not want my bar or this tragedy to be used to further any of their causes.”
Conspicuously, no photos of Esteve appeared in coverage of the UpStairs Lounge fire or its aftermath — and the bar owner also remained silent as he witnessed police looting the ashes of his business.
“Phil said the cash register, juke box, cigarette machine and some wallets had money removed,” recounted Esteve’s friend Bob McAnear, a former U.S. Customs officer. “Phil wouldn’t report it because, if he did, police would never allow him to operate a bar in New Orleans again.”
The next day, gay bar owners, incensed at declining gay bar traffic amid an atmosphere of anxiety, confronted Perry at a clandestine meeting. “How dare you hold your damn news conferences!” one business owner shouted.
Ignoring calls for gay self-censorship, Perry held a 250-person memorial for the fire victims the following Sunday, July 1, culminating in mourners defiantly marching out the front door of a French Quarter church into waiting news cameras. “Reverend Troy Perry awoke several sleeping giants, me being one of them,” recalled Charlene Schneider, a lesbian activist who walked out of that front door with Perry.

Esteve doubted the UpStairs Lounge story’s capacity to rouse gay political fervor. As the coroner buried four of his former patrons anonymously on the edge of town, Esteve quietly collected at least $25,000 in fire insurance proceeds. Less than a year later, he used the money to open another gay bar called the Post Office, where patrons of the UpStairs Lounge — some with visible burn scars — gathered but were discouraged from singing “United We Stand.”
New Orleans cops neglected to question the chief arson suspect and closed the investigation without answers in late August 1973. Gay elites in the city’s power structure began gaslighting the mourners who marched with Perry into the news cameras, casting suspicion on their memories and re-characterizing their moment of liberation as a stunt.
When a local gay journalist asked in April 1977, “Where are the gay activists in New Orleans?,” Esteve responded that there were none, because none were needed. “We don’t feel we’re discriminated against,” Esteve said. “New Orleans gays are different from gays anywhere else… Perhaps there is some correlation between the amount of gay activism in other cities and the degree of police harassment.”
An attitude of nihilism and disavowal descended upon the memory of the UpStairs Lounge victims, goaded by Esteve and fellow gay entrepreneurs who earned their keep via gay patrons drowning their sorrows each night instead of protesting the injustices that kept them drinking.
Into the 1980s, the story of the UpStairs Lounge all but vanished from conversation — with the exception of a few sanctuaries for gay political debate such as the local lesbian bar Charlene’s, run by the activist Charlene Schneider.
By 1988, the 15th anniversary of the fire, the UpStairs Lounge narrative comprised little more than a call for better fire codes and indoor sprinklers. UpStairs Lounge survivor Stewart Butler summed it up: “A tragedy that, as far as I know, no good came of.”
Finally, in 1991, at Stewart Butler and Charlene Schneider’s nudging, the UpStairs Lounge story became aligned with the crusade of liberated gays and lesbians seeking equal rights in Louisiana. The halls of power responded with intermittent progress. The New Orleans City Council, horrified by the story but not yet ready to take its look in the mirror, enacted an anti-discrimination ordinance protecting gays and lesbians in housing, employment, and public accommodations that Dec. 12 — more than 18 years after the fire.
“I believe the fire was the catalyst for the anger to bring us all to the table,” Schneider told The Times-Picayune, a tacit rebuke to Esteve’s strategy of silent accommodation. Even Esteve seemed to change his stance with time, granting a full interview with the first UpStairs Lounge scholar Johnny Townsend sometime around 1989.
Most of the figures in this historic tale are now deceased. What’s left is an enduring story that refused to go gently. The story now echoes around the world — a musical about the UpStairs Lounge fire recently played in Tokyo, translating the gay underworld of the 1973 French Quarter for Japanese audiences.
When I finished my presentation to the City Council last June, I looked up to see the seven council members in tears. Unanimously, they approved a resolution acknowledging the historic failures of city leaders in the wake of the UpStairs Lounge fire.
Council members personally apologized to UpStairs Lounge families and survivors seated in the chamber in a symbolic act that, though it could not bring back those who died, still mattered greatly to those whose pain had been denied, leaving them to grieve alone. At long last, official silence and indifference gave way to heartfelt words of healing.
The way Americans remember the past is an active, ongoing process. Our collective memory is malleable, but it matters because it speaks volumes about our maturity as a people, how we acknowledge the past’s influence in our lives, and how it shapes the examples we set for our youth. Do we grapple with difficult truths, or do we duck accountability by defaulting to nostalgia and bluster? Or worse, do we simply ignore the past until it fades into a black hole of ignorance and indifference?
I believe that a factual retelling of the UpStairs Lounge tragedy — and how, 50 years onward, it became known internationally — resonates beyond our current divides. It reminds queer and non-queer Americans that ignoring the past holds back the present, and that silence is no cure for what ails a participatory nation.
Silence isolates. Silence gaslights and shrouds. It preserves the power structures that scapegoat the disempowered.
Solidarity, on the other hand, unites. Solidarity illuminates a path forward together. Above all, solidarity transforms the downtrodden into a resounding chorus of citizens — in the spirit of voices who once gathered ‘round a white baby grand piano and sang, joyfully and loudly, “United We Stand.”

Robert W. Fieseler is a New Orleans-based journalist and the author of “Tinderbox: the Untold Story of the Up Stairs Lounge Fire and the Rise of Gay Liberation.”
homepage news
New Supreme Court term includes critical LGBTQ case with ‘terrifying’ consequences
Business owner seeks to decline services for same-sex weddings

The U.S. Supreme Court, after a decision overturning Roe v. Wade that still leaves many reeling, is starting a new term with justices slated to revisit the issue of LGBTQ rights.
In 303 Creative v. Elenis, the court will return to the issue of whether or not providers of custom-made goods can refuse service to LGBTQ customers on First Amendment grounds. In this case, the business owner is Lorie Smith, a website designer in Colorado who wants to opt out of providing her graphic design services for same-sex weddings despite the civil rights law in her state.
Jennifer Pizer, acting chief legal officer of Lambda Legal, said in an interview with the Blade, “it’s not too much to say an immeasurably huge amount is at stake” for LGBTQ people depending on the outcome of the case.
“This contrived idea that making custom goods, or offering a custom service, somehow tacitly conveys an endorsement of the person — if that were to be accepted, that would be a profound change in the law,” Pizer said. “And the stakes are very high because there are no practical, obvious, principled ways to limit that kind of an exception, and if the law isn’t clear in this regard, then the people who are at risk of experiencing discrimination have no security, no effective protection by having a non-discrimination laws, because at any moment, as one makes their way through the commercial marketplace, you don’t know whether a particular business person is going to refuse to serve you.”
The upcoming arguments and decision in the 303 Creative case mark a return to LGBTQ rights for the Supreme Court, which had no lawsuit to directly address the issue in its previous term, although many argued the Dobbs decision put LGBTQ rights in peril and threatened access to abortion for LGBTQ people.
And yet, the 303 Creative case is similar to other cases the Supreme Court has previously heard on the providers of services seeking the right to deny services based on First Amendment grounds, such as Masterpiece Cakeshop and Fulton v. City of Philadelphia. In both of those cases, however, the court issued narrow rulings on the facts of litigation, declining to issue sweeping rulings either upholding non-discrimination principles or First Amendment exemptions.
Pizer, who signed one of the friend-of-the-court briefs in opposition to 303 Creative, said the case is “similar in the goals” of the Masterpiece Cakeshop litigation on the basis they both seek exemptions to the same non-discrimination law that governs their business, the Colorado Anti-Discrimination Act, or CADA, and seek “to further the social and political argument that they should be free to refuse same-sex couples or LGBTQ people in particular.”
“So there’s the legal goal, and it connects to the social and political goals and in that sense, it’s the same as Masterpiece,” Pizer said. “And so there are multiple problems with it again, as a legal matter, but also as a social matter, because as with the religion argument, it flows from the idea that having something to do with us is endorsing us.”
One difference: the Masterpiece Cakeshop litigation stemmed from an act of refusal of service after owner, Jack Phillips, declined to make a custom-made wedding cake for a same-sex couple for their upcoming wedding. No act of discrimination in the past, however, is present in the 303 Creative case. The owner seeks to put on her website a disclaimer she won’t provide services for same-sex weddings, signaling an intent to discriminate against same-sex couples rather than having done so.
As such, expect issues of standing — whether or not either party is personally aggrieved and able bring to a lawsuit — to be hashed out in arguments as well as whether the litigation is ripe for review as justices consider the case. It’s not hard to see U.S. Chief Justice John Roberts, who has sought to lead the court to reach less sweeping decisions (sometimes successfully, and sometimes in the Dobbs case not successfully) to push for a decision along these lines.
Another key difference: The 303 Creative case hinges on the argument of freedom of speech as opposed to the two-fold argument of freedom of speech and freedom of religious exercise in the Masterpiece Cakeshop litigation. Although 303 Creative requested in its petition to the Supreme Court review of both issues of speech and religion, justices elected only to take up the issue of free speech in granting a writ of certiorari (or agreement to take up a case). Justices also declined to accept another question in the petition request of review of the 1990 precedent in Smith v. Employment Division, which concluded states can enforce neutral generally applicable laws on citizens with religious objections without violating the First Amendment.
Representing 303 Creative in the lawsuit is Alliance Defending Freedom, a law firm that has sought to undermine civil rights laws for LGBTQ people with litigation seeking exemptions based on the First Amendment, such as the Masterpiece Cakeshop case.
Kristen Waggoner, president of Alliance Defending Freedom, wrote in a Sept. 12 legal brief signed by her and other attorneys that a decision in favor of 303 Creative boils down to a clear-cut violation of the First Amendment.
“Colorado and the United States still contend that CADA only regulates sales transactions,” the brief says. “But their cases do not apply because they involve non-expressive activities: selling BBQ, firing employees, restricting school attendance, limiting club memberships, and providing room access. Colorado’s own cases agree that the government may not use public-accommodation laws to affect a commercial actor’s speech.”
Pizer, however, pushed back strongly on the idea a decision in favor of 303 Creative would be as focused as Alliance Defending Freedom purports it would be, arguing it could open the door to widespread discrimination against LGBTQ people.
“One way to put it is art tends to be in the eye of the beholder,” Pizer said. “Is something of a craft, or is it art? I feel like I’m channeling Lily Tomlin. Remember ‘soup and art’? We have had an understanding that whether something is beautiful or not is not the determining factor about whether something is protected as artistic expression. There’s a legal test that recognizes if this is speech, whose speech is it, whose message is it? Would anyone who was hearing the speech or seeing the message understand it to be the message of the customer or of the merchants or craftsmen or business person?”
Despite the implications in the case for LGBTQ rights, 303 Creative may have supporters among LGBTQ people who consider themselves proponents of free speech.
One joint friend-of-the-court brief before the Supreme Court, written by Dale Carpenter, a law professor at Southern Methodist University who’s written in favor of LGBTQ rights, and Eugene Volokh, a First Amendment legal scholar at the University of California, Los Angeles, argues the case is an opportunity to affirm the First Amendment applies to goods and services that are uniquely expressive.
“Distinguishing expressive from non-expressive products in some contexts might be hard, but the Tenth Circuit agreed that Smith’s product does not present a hard case,” the brief says. “Yet that court (and Colorado) declined to recognize any exemption for products constituting speech. The Tenth Circuit has effectively recognized a state interest in subjecting the creation of speech itself to antidiscrimination laws.”
Oral arguments in the case aren’t yet set, but may be announced soon. Set to defend the state of Colorado and enforcement of its non-discrimination law in the case is Colorado Solicitor General Eric Reuel Olson. Just this week, the U.S. Supreme Court announced it would grant the request to the U.S. solicitor general to present arguments before the justices on behalf of the Biden administration.
With a 6-3 conservative majority on the court that has recently scrapped the super-precedent guaranteeing the right to abortion, supporters of LGBTQ rights may think the outcome of the case is all but lost, especially amid widespread fears same-sex marriage would be next on the chopping block. After the U.S. Tenth Circuit Court of Appeals ruled against 303 Creative in the lawsuit, the simple action by the Supreme Court to grant review in the lawsuit suggests they are primed to issue a reversal and rule in favor of the company.
Pizer, acknowledging the call to action issued by LGBTQ groups in the aftermath of the Dobbs decision, conceded the current Supreme Court issuing the ruling in this case is “a terrifying prospect,” but cautioned the issue isn’t so much the makeup of the court but whether or not justices will continue down the path of abolishing case law.
“I think the question that we’re facing with respect to all of the cases or at least many of the cases that are in front of the court right now, is whether this court is going to continue on this radical sort of wrecking ball to the edifice of settled law and seemingly a goal of setting up whole new structures of what our basic legal principles are going to be. Are we going to have another term of that?” Pizer said. “And if so, that’s terrifying.”
homepage news
Kelley Robinson, a Black, queer woman, named president of Human Rights Campaign
Progressive activist a veteran of Planned Parenthood Action Fund

Kelley Robinson, a Black, queer woman and veteran of Planned Parenthood Action Fund, is to become the next president of the Human Rights Campaign, the nation’s leading LGBTQ group announced on Tuesday.
Robinson is set to become the ninth president of the Human Rights Campaign after having served as executive director of Planned Parenthood Action Fund and more than 12 years of experience as a leader in the progressive movement. She’ll be the first Black, queer woman to serve in that role.
“I’m honored and ready to lead HRC — and our more than three million member-advocates — as we continue working to achieve equality and liberation for all Lesbian, Gay, Bisexual, Transgender, and Queer people,” Robinson said. “This is a pivotal moment in our movement for equality for LGBTQ+ people. We, particularly our trans and BIPOC communities, are quite literally in the fight for our lives and facing unprecedented threats that seek to destroy us.”
The next Human Rights Campaign president is named as Democrats are performing well in polls in the mid-term elections after the U.S. Supreme Court overturned Roe v. Wade, leaving an opening for the LGBTQ group to play a key role amid fears LGBTQ rights are next on the chopping block.
“The overturning of Roe v. Wade reminds us we are just one Supreme Court decision away from losing fundamental freedoms including the freedom to marry, voting rights, and privacy,” Robinson said. “We are facing a generational opportunity to rise to these challenges and create real, sustainable change. I believe that working together this change is possible right now. This next chapter of the Human Rights Campaign is about getting to freedom and liberation without any exceptions — and today I am making a promise and commitment to carry this work forward.”
The Human Rights Campaign announces its next president after a nearly year-long search process after the board of directors terminated its former president Alphonso David when he was ensnared in the sexual misconduct scandal that led former New York Gov. Andrew Cuomo to resign. David has denied wrongdoing and filed a lawsuit against the LGBTQ group alleging racial discrimination.

-

 State Department2 days ago
State Department2 days agoState Department releases annual human rights report
-

 Maryland4 days ago
Maryland4 days agoJoe Vogel campaign holds ‘Big Gay Canvass Kickoff’
-

 Politics3 days ago
Politics3 days agoSmithsonian staff concerned about future of LGBTQ programming amid GOP scrutiny
-

 The White House1 day ago
The White House1 day agoWhite House debuts action plan targeting pollutants in drinking water












